The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For . The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. The correct answer is b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. As a result, the electron affinity of cl ion is equal in magnitude to the ionization. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The correct answer is option b: In our case, the ion formed from the ionization process is cl. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The clion the ci+ ion d. The ionization energy of the cl atom is equal in. 25) choose the best response for the following:
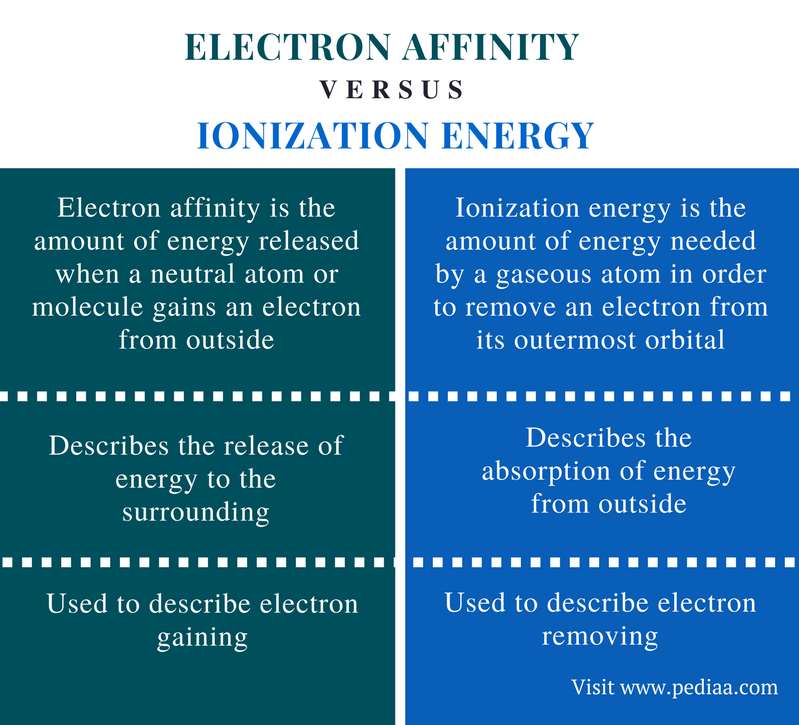
from pediaa.com
The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The ionization energy of the cl atom is equal in. The clion the ci+ ion d. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. 25) choose the best response for the following: The correct answer is option b: In our case, the ion formed from the ionization process is cl. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. The correct answer is b:
Difference Between Electron Affinity and Ionization Energy Definition
The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For In our case, the ion formed from the ionization process is cl. In our case, the ion formed from the ionization process is cl. The clion the ci+ ion d. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The correct answer is option b: As a result, the electron affinity of cl ion is equal in magnitude to the ionization. 25) choose the best response for the following: The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The ionization energy of the cl atom is equal in. The correct answer is b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion.
From www.slideserve.com
PPT Periodic Patterns PowerPoint Presentation, free download ID6476349 The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The correct answer is option b: The correct answer is b: As a result, the electron affinity of cl ion is equal in magnitude to the ionization. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The correct answer is option b: The ionization energy of the cl atom is equal in. In our case, the ion formed from the ionization process is cl. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From askfilo.com
Ionisation energy of F−is equal in magnitude with 8. Electron affinity is.. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For In our case, the ion formed from the ionization process is cl. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. The clion the ci+ ion d. The ionization energy for the chlorine atom is equal in magnitude to the electron. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From periodictableguide.com
Periodic table with Ionization Energy Values (Labeled Image) The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The correct answer is b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The correct answer is option b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. As a result, the electron affinity of cl ion is equal in magnitude to the ionization.. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.toppr.com
In the formation of a chloride ion, from an isolated gaseous chlorine The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The correct answer is option b: In our case, the ion formed from the ionization process is cl. The ionization energy of a chlorine atom is. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.numerade.com
SOLVEDChoose the best response for the following. The ionization The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The correct answer is option b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion,. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.numerade.com
Cesium has the smallest ionization energy of all elements (376 kJ/mol The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. As a result, the electron affinity of cl ion is equal in magnitude to the ionization. The clion the ci+ ion d. The. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.youtube.com
Chapter 03 23 Electron Affinity vs Ionization Energy YouTube The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy of the cl atom is equal in. As a result, the electron affinity of cl ion is equal in magnitude to the ionization. In our case, the ion formed from the ionization process is cl. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The correct answer is b:. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy of the cl atom is equal in. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. In our case, the ion formed from the ionization process is cl. As a result, the electron affinity of cl ion is. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.pinterest.com
Periodic Trend Electron affinity, Ionization energy, Modern periodic The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The clion the ci+ ion d. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. The correct answer is b: 25) choose the best response for the following: The ionization energy for the chlorine atom is equal in magnitude to the. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The clion the ci+ ion d. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. As a result, the electron affinity. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From general.chemistrysteps.com
Ionization energy Chemistry Steps The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The ionization energy for the chlorine. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For 25) choose the best response for the following: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. The ionization energy of the cl atom is equal in. The correct answer is option b: The correct answer is b: In our case, the ion formed from the ionization process is. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.youtube.com
Electron Affinity Trend, Basic Introduction, Chemistry YouTube The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For As a result, the electron affinity of cl ion is equal in magnitude to the ionization. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. The ionization energy of the cl atom is equal in. The ionization energy for the chlorine atom is equal in magnitude to the electron. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For In our case, the ion formed from the ionization process is cl. The correct answer is option b: The clion the ci+ ion d. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. As a result, the electron affinity of cl ion is equal in magnitude to the ionization. 25) choose the best. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From general.chemistrysteps.com
Ionization energy Chemistry Steps The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. In our case, the ion formed from the ionization process is cl. The correct answer is b: The ionization energy of the cl atom is equal in. The ionization energy for the. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From alevelchemistry.co.uk
Ionisation Energy ALevel Chemistry Revision Notes The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The ionization energy of the cl atom is equal in.. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From general.chemistrysteps.com
Ionization energy Chemistry Steps The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For In our case, the ion formed from the ionization process is cl. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. As a result,. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The correct answer is b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. 25) choose the best response for the following: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The ionization energy of the cl atom is equal in. In our case, the. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.numerade.com
SOLVED QUESTION 5 (Start on a new page. The first ionisation energy The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The clion the ci+ ion d. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The correct answer is option b: As a result, the electron affinity of cl ion is equal in magnitude to the. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.slideserve.com
PPT Ionization Energy PowerPoint Presentation, free download ID6548427 The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For As a result, the electron affinity of cl ion is equal in magnitude to the ionization. The correct answer is option b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.britannica.com
Ionization energy Definition & Facts Britannica The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The correct answer is option b: The ionization energy of the cl atom is equal in. 25) choose the best response for the following: The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy change. In our case, the ion formed from the. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The clion the ci+ ion d. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy of the cl atom is equal in. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have equal energy. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From pediaa.com
Difference Between Electron Affinity and Ionization Energy Definition The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The clion the ci+ ion d. 25) choose the best response for the following: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. The correct answer is option b: The ionization energy. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.numerade.com
SOLVED Choose the best response for the following. The ionization The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For 25) choose the best response for the following: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. In our case, the ion formed from the ionization process is cl. The ionization energy of the cl atom. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For 25) choose the best response for the following: In our case, the ion formed from the ionization process is cl. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. The clion the ci+ ion d. The correct answer is option b: The correct answer is b: The ionization energy. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.numerade.com
SOLVED 26. Select the correct statement Ionization energy and metallic The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy of the cl atom is equal in. In our case, the ion formed from the ionization process is cl. The clion the ci+ ion d. The correct answer is option b: As a result, the electron affinity of cl ion is equal in magnitude to the ionization. The correct answer is b: 25) choose the best response. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The correct answer is b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy of the cl atom is equal in. In our case, the ion formed from the ionization process is cl. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.toppr.com
In the formation of a chloride ion from an isolated gaseous chlorine The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For 25) choose the best response for the following: The correct answer is b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. As a result, the electron affinity of cl ion is equal in magnitude. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.slideserve.com
PPT Ionization Energy and Electron Affinity PowerPoint Presentation The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The correct answer is option b: In our case, the ion formed from the ionization process is cl. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes have. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.chegg.com
Solved Understanding the definitions of ionization energy The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. The ionization energy of the cl atom is equal in. The correct answer is b: The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The clion the ci+ ion d. The ionization energy for the chlorine. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. The ionization energy of a chlorine atom is equal in magnitude to the electron affinity of a c l + ion, as both processes. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.numerade.com
SOLVED Choose the best response for the following. The ionization The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The correct answer is b: The clion the ci+ ion d. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for a. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for the cl − ion. In our case, the ion formed from the ionization process is cl.. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For The clion the ci+ ion d. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for 1. In our case, the ion formed from the ionization process is cl. The ionization energy of the cl atom is equal in. The correct answer is b: 25) choose the best response for the following: The correct. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.
From www.pinterest.co.kr
Electron Affinity Trend and Definition Electron affinity, Ionization The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For In our case, the ion formed from the ionization process is cl. The correct answer is b: 25) choose the best response for the following: The correct answer is option b: The clion the ci+ ion d. The ionization energy of the cl atom is equal in. The ionization energy for the chlorine atom is equal in magnitude to the. The Ionization Energy For The Chlorine Atom Is Equal In Magnitude To The Electron Affinity For.