Precision In Pharmaceutical Analysis . In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). Validation in pharmaceutical analysis part ii: This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). 123 successful validation of a. Central importance of precision to establish acceptance criteria and for verifying and.
from precisionmedicinealliance.org
The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. Central importance of precision to establish acceptance criteria and for verifying and. Validation in pharmaceutical analysis part ii: This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). 123 successful validation of a. In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,.
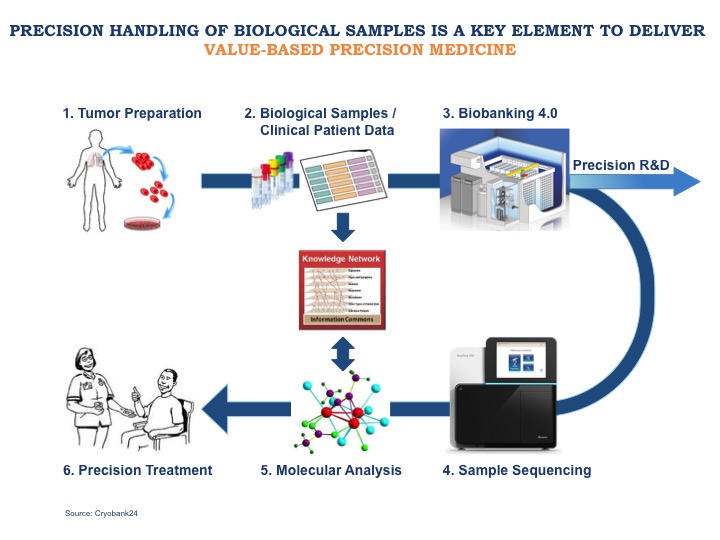
PRECISION HANDLING Precision Medicine Alliance
Precision In Pharmaceutical Analysis Validation in pharmaceutical analysis part ii: This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). Validation in pharmaceutical analysis part ii: Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. Central importance of precision to establish acceptance criteria and for verifying and. In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. 123 successful validation of a. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output).
From pharmaceuticalcarrier.com
Standard Operating Procedure (SOP) on Customized HPLC Procedures for Precision In Pharmaceutical Analysis This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. In pharmaceutical analysis, the samples are typically finished pharmaceutical products,. Precision In Pharmaceutical Analysis.
From www.mdpi.com
BDCC Free FullText Artificial Intelligence in Pharmaceutical and Precision In Pharmaceutical Analysis Central importance of precision to establish acceptance criteria and for verifying and. Validation in pharmaceutical analysis part ii: The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. 123 successful validation of a. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center. Precision In Pharmaceutical Analysis.
From proleantech.com
Precision VS Accuracy in Machining Difference & Importance Precision In Pharmaceutical Analysis The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). 123 successful validation of a. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during. Precision In Pharmaceutical Analysis.
From www.americanpharmaceuticalreview.com
Analytical Method Validation for Quality Assurance and Process Precision In Pharmaceutical Analysis The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Central importance of precision to establish acceptance criteria and for verifying and. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. The multivariate calibration model relate the input data to a value for 122. Precision In Pharmaceutical Analysis.
From www.healthcatalyst.com
DataDriven Precision Medicine Individualizes Treatment Precision In Pharmaceutical Analysis Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). The multivariate calibration model relate the input data to a. Precision In Pharmaceutical Analysis.
From www.frontiersin.org
Frontiers Towards Building a Quantitative Proteomics Toolbox in Precision In Pharmaceutical Analysis 123 successful validation of a. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). In pharmaceutical analysis, the samples. Precision In Pharmaceutical Analysis.
From precisionmedicinealliance.org
PRECISION HANDLING Precision Medicine Alliance Precision In Pharmaceutical Analysis 123 successful validation of a. Central importance of precision to establish acceptance criteria and for verifying and. The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the. Precision In Pharmaceutical Analysis.
From procure-net.com
Trometamol EP Reference Standard An Essential Product for Precision Precision In Pharmaceutical Analysis 123 successful validation of a. In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. Central importance of precision to establish acceptance criteria and for verifying and. This guidance has been prepared by the office of. Precision In Pharmaceutical Analysis.
From procure-net.com
NFormylphenylephrine Pharmaceutical Secondary Standard A Certified Precision In Pharmaceutical Analysis Central importance of precision to establish acceptance criteria and for verifying and. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and. Precision In Pharmaceutical Analysis.
From chinainstrument.en.made-in-china.com
High Precision High Performance Liquid Chromatograph Used in Precision In Pharmaceutical Analysis 123 successful validation of a. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). Accuracy and precision are the. Precision In Pharmaceutical Analysis.
From www.mdpi.com
IJMS Free FullText Precision Medicine Approaches with Metabolomics Precision In Pharmaceutical Analysis 123 successful validation of a. In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample.. Precision In Pharmaceutical Analysis.
From www.usfhealthonline.com
Precision Medicine and Informatics Precision In Pharmaceutical Analysis In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Central importance of precision to establish acceptance criteria and for verifying and. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and. Precision In Pharmaceutical Analysis.
From procure-net.com
Rosiglitazone Related Compound A USP Reference Standard Precision in Precision In Pharmaceutical Analysis Central importance of precision to establish acceptance criteria and for verifying and. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and. Precision In Pharmaceutical Analysis.
From dpsru.edu.in
Delhi Pharmaceutical Sciences and Research University Precision In Pharmaceutical Analysis In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. 123 successful validation of a. Validation in pharmaceutical analysis part ii: The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). Central importance of precision to establish acceptance criteria and for verifying and. This guidance has. Precision In Pharmaceutical Analysis.
From procure-net.com
HighQuality Aminoglutethimide Impurity D British Pharmacopoeia Precision In Pharmaceutical Analysis Central importance of precision to establish acceptance criteria and for verifying and. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and. Precision In Pharmaceutical Analysis.
From www.cell.com
Dietary interventions and precision nutrition in cancer therapy Trends Precision In Pharmaceutical Analysis 123 successful validation of a. Validation in pharmaceutical analysis part ii: In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). Central importance of precision to establish acceptance criteria and for verifying and. Accuracy and precision. Precision In Pharmaceutical Analysis.
From pnbvesper.com
The Role of Molecule Research in India’s Pharmaceutical Landscape Precision In Pharmaceutical Analysis This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. 123 successful validation of a. Accuracy and precision are the most important criteria in the assessment of. Precision In Pharmaceutical Analysis.
From www.frontiersin.org
Frontiers Data Integration Challenges for Machine Learning in Precision In Pharmaceutical Analysis This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. 123 successful validation of a. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic,. Precision In Pharmaceutical Analysis.
From www.contata.com
Transformative Text Analysis in Pharmaceutical Research Precision In Pharmaceutical Analysis Validation in pharmaceutical analysis part ii: 123 successful validation of a. In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Central importance of precision to establish acceptance criteria and for verifying and. The multivariate calibration model relate the input data to a. Precision In Pharmaceutical Analysis.
From www.youtube.com
Bioinformatics for Precision Medicine Session 1, June 16th, 2020 YouTube Precision In Pharmaceutical Analysis This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). In pharmaceutical analysis, the samples are typically finished pharmaceutical products,. Precision In Pharmaceutical Analysis.
From oxfordglobal.com
Harmonized MultiOmics Data Analysis How Does It Impact Precision In Pharmaceutical Analysis Central importance of precision to establish acceptance criteria and for verifying and. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. 123 successful validation of a.. Precision In Pharmaceutical Analysis.
From www.chromatographyonline.com
Validation of StabilityIndicating HPLC Methods for Pharmaceuticals Precision In Pharmaceutical Analysis Validation in pharmaceutical analysis part ii: The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). 123 successful validation of a. In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Central. Precision In Pharmaceutical Analysis.
From wayeal.en.made-in-china.com
Wayeal Precision Laboratory Ion Chromatograph Used in Pharmaceutical Precision In Pharmaceutical Analysis In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. Validation in pharmaceutical analysis part ii: Central importance of precision to establish acceptance criteria and for verifying and. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. The review highlights a variety of analytical techniques. Precision In Pharmaceutical Analysis.
From www.investopedia.com
Evaluating Pharmaceutical Companies Precision In Pharmaceutical Analysis This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). 123 successful validation of a. Central importance of precision to establish acceptance criteria and for verifying and. Accuracy and precision are the most important criteria in the assessment of an analytical. Precision In Pharmaceutical Analysis.
From medicalfuturist.com
Top Companies Using A.I. In Drug Discovery And Development Precision In Pharmaceutical Analysis 123 successful validation of a. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Validation in pharmaceutical analysis part ii: Central importance of precision to establish acceptance criteria and for verifying and. The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). Accuracy and. Precision In Pharmaceutical Analysis.
From www.ucd.ie
MSc in Data Analytics for Precision Medicine UCD Health Affairs Precision In Pharmaceutical Analysis Validation in pharmaceutical analysis part ii: 123 successful validation of a. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. The multivariate calibration model relate the input data to a value for 122 the. Precision In Pharmaceutical Analysis.
From www.velp.com
EVERYTHING YOU NEED TO ACHIEVE THE HIGHEST PRECISION AND ACCURACY IN Precision In Pharmaceutical Analysis The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). 123 successful validation of a. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Central importance of precision to establish acceptance criteria and for verifying and. Accuracy and precision are the most important criteria. Precision In Pharmaceutical Analysis.
From www.iomcworld.org
Journal of Pharmaceutical Sciences and Drug Development iomc Precision In Pharmaceutical Analysis 123 successful validation of a. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). Validation in pharmaceutical analysis part. Precision In Pharmaceutical Analysis.
From www.youtube.com
Accuracy and Precision Definition Determination Pharmaceutical Precision In Pharmaceutical Analysis In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. Validation in pharmaceutical analysis part ii: Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder). Precision In Pharmaceutical Analysis.
From procure-net.com
Supelco Loss on Drying Proficiency Testing (PT) Highprecision Precision In Pharmaceutical Analysis Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. Central importance of precision to establish acceptance criteria and for verifying and. Validation in pharmaceutical analysis part ii: This guidance has been prepared by the. Precision In Pharmaceutical Analysis.
From www.cell.com
Enabling Technologies for Personalized and Precision Medicine Trends Precision In Pharmaceutical Analysis This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). The review highlights a variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic,. 123 successful validation of a. Validation in pharmaceutical analysis part ii: Central importance of precision to establish. Precision In Pharmaceutical Analysis.
From international.blogs.hopkinsmedicine.org
The Time Is Now for Precision Care Global Promise Precision In Pharmaceutical Analysis This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). 123 successful validation of a. Central importance of precision to. Precision In Pharmaceutical Analysis.
From www.youtube.com
CLASSIFICATION OF ANALYTICAL METHODS IN PHARMACEUTICAL ANALYSIS Precision In Pharmaceutical Analysis The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). 123 successful validation of a. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and research (cder) and the center for biologics evaluation and research (cber). In pharmaceutical analysis, the samples. Precision In Pharmaceutical Analysis.
From www.ama-assn.org
Find clarity in precision medicine American Medical Association Precision In Pharmaceutical Analysis Central importance of precision to establish acceptance criteria and for verifying and. Accuracy and precision are the most important criteria in the assessment of an analytical method, and monitoring quality control during sample. The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). This guidance has been prepared by. Precision In Pharmaceutical Analysis.
From www.slideserve.com
PPT Pharmaceutical Development PowerPoint Presentation, free download Precision In Pharmaceutical Analysis The multivariate calibration model relate the input data to a value for 122 the property of interest (i.e., the model output). In pharmaceutical analysis, the samples are typically finished pharmaceutical products, biological samples, impurities,. 123 successful validation of a. Validation in pharmaceutical analysis part ii: Accuracy and precision are the most important criteria in the assessment of an analytical method,. Precision In Pharmaceutical Analysis.