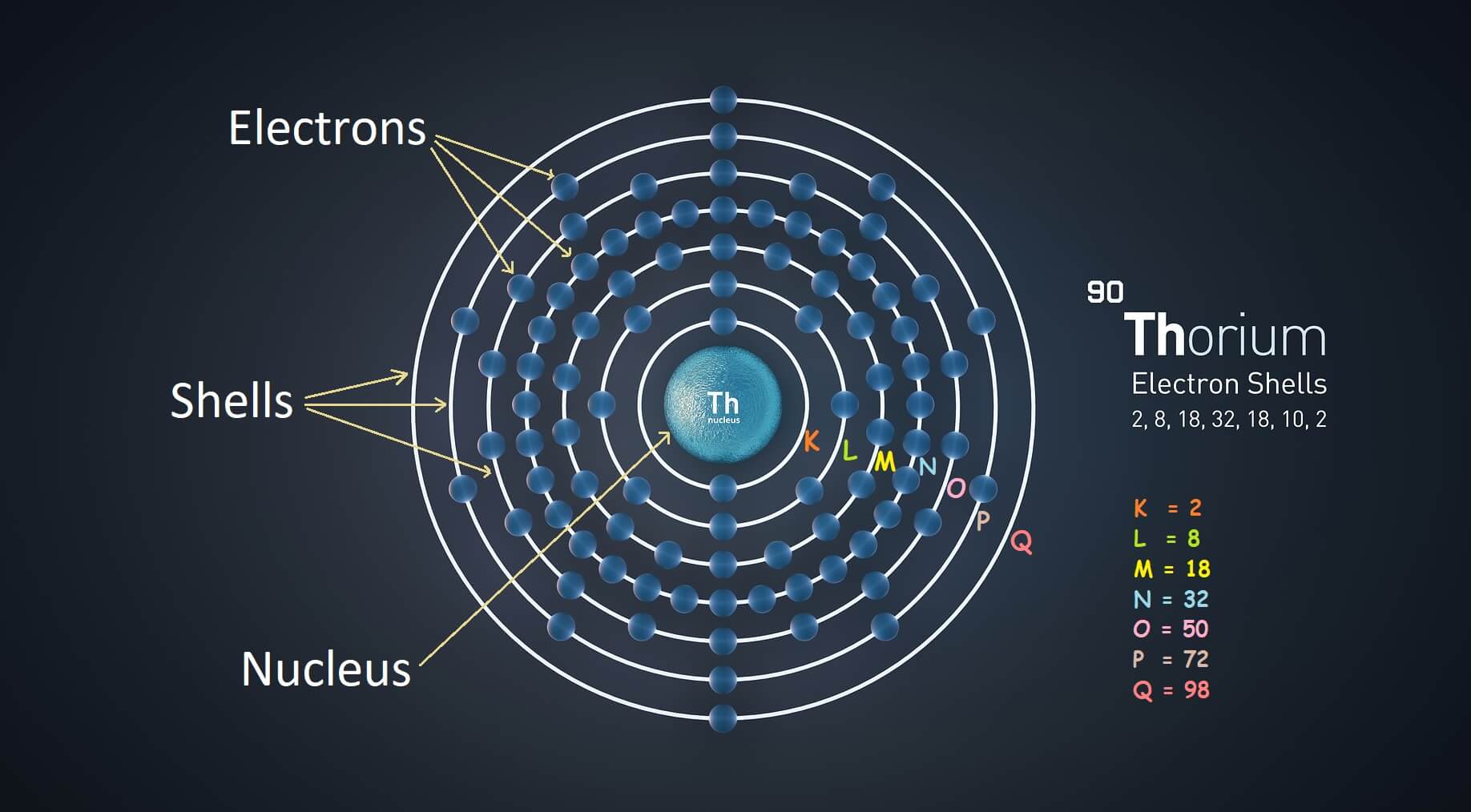
Electron Shell Configuration S P D F . The electron configuration in the picture above tells us that the electrons are. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. These line groups are called sharp, principal, diffuse, and fundamental. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its. The orbital names s, p, d, and f describe electron configuration. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The orbital letters are associated with the angular. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell.
from newtondesk.com
The electron configuration in the picture above tells us that the electrons are. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. The orbital letters are associated with the angular. The orbital names s, p, d, and f describe electron configuration. These line groups are called sharp, principal, diffuse, and fundamental. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant.
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry
Electron Shell Configuration S P D F The orbital names s, p, d, and f describe electron configuration. The electron configuration in the picture above tells us that the electrons are. These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its. The orbital letters are associated with the angular. The orbital names s, p, d, and f describe electron configuration. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell.
From www.youtube.com
Electron Configurations SPDF and Kernel Notation Notes YouTube Electron Shell Configuration S P D F The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. The orbital names s, p, d, and f describe electron configuration. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration. Electron Shell Configuration S P D F.
From www.chem.fsu.edu
Electron Configurations Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The orbital names s, p, d, and f describe electron configuration. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones. Electron Shell Configuration S P D F.
From unacademy.com
Notes on the Irregularites of Electronic Configuration of Transition Metals Electron Shell Configuration S P D F The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The orbital. Electron Shell Configuration S P D F.
From chemwiki.ucdavis.edu
Electronic Configurations Chemwiki Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration in the picture above tells us that the electrons are. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. To write the electron configuration of an atom, identify the energy level of interest and write the number. Electron Shell Configuration S P D F.
From www.youtube.com
Electronic Configuration of a Atom Atomic Structure Electronic Electron Shell Configuration S P D F In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The electron configuration in the picture above tells us that the electrons are. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The orbital names s, p, d,. Electron Shell Configuration S P D F.
From www.thoughtco.com
Electron Configuration Chart Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. The orbital names s, p, d, and f describe electron configuration. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. To write the electron configuration of an atom, identify the energy level of interest and write the. Electron Shell Configuration S P D F.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Electron Shell Configuration S P D F The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The orbital names s, p, d, and f describe electron configuration. The electron configuration in the picture above tells us that the electrons are. These line groups are called sharp, principal, diffuse, and fundamental. To write the electron. Electron Shell Configuration S P D F.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. The orbital letters are associated with the angular. The electron configuration in the picture above tells us that the electrons are. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The orbital names s, p, d, and. Electron Shell Configuration S P D F.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration in the picture above tells us that the electrons are. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The orbital letters are associated with the angular. The orbital names s, p, d, and f describe electron. Electron Shell Configuration S P D F.
From chem.libretexts.org
7.8B Electron Configurations and the Periodic Table Chemistry LibreTexts Electron Shell Configuration S P D F The electron configuration in the picture above tells us that the electrons are. The orbital letters are associated with the angular. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells. Electron Shell Configuration S P D F.
From www.britannica.com
Electron shell Definition & Facts Britannica Electron Shell Configuration S P D F The orbital letters are associated with the angular. These line groups are called sharp, principal, diffuse, and fundamental. The orbital names s, p, d, and f describe electron configuration. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its. The electron configuration in the. Electron Shell Configuration S P D F.
From mistrywww.kentchemistry.com
Electron Configurations and the Periodic Table Electron Shell Configuration S P D F In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its. The orbital names s, p, d, and f describe electron configuration. The electron configuration of. Electron Shell Configuration S P D F.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and Electron Shell Configuration S P D F The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its. In any atom with two or more electrons, the repulsion between the. Electron Shell Configuration S P D F.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration in the picture above tells us that the electrons are. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and. Electron Shell Configuration S P D F.
From gzscienceclassonline.weebly.com
1. Electron Configuration Electron Shell Configuration S P D F The orbital letters are associated with the angular. The orbital names s, p, d, and f describe electron configuration. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration of an element shows how electrons are distributed in. Electron Shell Configuration S P D F.
From chemistry291.blogspot.com
[5 Steps] Electronic Configuration of Fluorine(F) Electron Shell Configuration S P D F The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The electron configuration in the picture above tells us that the electrons are. These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration will tell you what energy level, subshell, and how many electrons occupy. Electron Shell Configuration S P D F.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube Electron Shell Configuration S P D F In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The electron configuration in the picture above tells us that the electrons are. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. To write the electron configuration of. Electron Shell Configuration S P D F.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration in the picture above tells us that the electrons are. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The orbital names s, p, d, and f describe electron configuration. The electron configuration will. Electron Shell Configuration S P D F.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Electron Shell Configuration S P D F In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The orbital names s, p, d, and f describe electron configuration. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. The electron configuration in the picture above tells us that the electrons are.. Electron Shell Configuration S P D F.
From www.animalia-life.club
Electron Shell Diagram Electron Shell Configuration S P D F The electron configuration in the picture above tells us that the electrons are. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. These line groups are called sharp, principal, diffuse, and fundamental. To write the electron configuration of an atom, identify the energy level of interest and write the number. Electron Shell Configuration S P D F.
From www.breakingatom.com
Advanced Electron Configuration Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The orbital letters are associated with the angular. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons. Electron Shell Configuration S P D F.
From ar.inspiredpencil.com
Electron Orbitals S P D F Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. In any atom with two or more electrons, the repulsion between. Electron Shell Configuration S P D F.
From sciencenotes.org
List of Electron Configurations of Elements Electron Shell Configuration S P D F In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The orbital letters are associated with the angular. The orbital names s, p, d, and f describe electron configuration.. Electron Shell Configuration S P D F.
From courses.lumenlearning.com
3.4 Electronic Structure of Atoms (Electron Configurations) General Electron Shell Configuration S P D F The orbital letters are associated with the angular. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. In any atom with two or more electrons, the repulsion between the electrons. Electron Shell Configuration S P D F.
From saylordotorg.github.io
Organization of Electrons in Atoms Electron Shell Configuration S P D F The electron configuration in the picture above tells us that the electrons are. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. The orbital letters are associated with the angular. The orbital names s, p, d, and f describe electron configuration. To write the electron configuration of an atom, identify the energy. Electron Shell Configuration S P D F.
From www.animalia-life.club
Electron Shell Diagram Electron Shell Configuration S P D F In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its. The electron configuration in the picture above tells us that the electrons are. These line. Electron Shell Configuration S P D F.
From pages.swcp.com
Parsing the spdf electron orbital model Electron Shell Configuration S P D F The orbital letters are associated with the angular. The orbital names s, p, d, and f describe electron configuration. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. To write the electron configuration. Electron Shell Configuration S P D F.
From chemwiki.ucdavis.edu
Electron Configuration of Transition Metals Chemwiki Electron Shell Configuration S P D F The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The orbital. Electron Shell Configuration S P D F.
From chemistrytalk.org
Electron Shells ChemTalk Electron Shell Configuration S P D F The orbital letters are associated with the angular. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The orbital names s, p, d, and f describe electron configuration.. Electron Shell Configuration S P D F.
From byjus.com
What is the value of s,p,d.f? Electron Shell Configuration S P D F The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The orbital letters are associated with the angular. The electron configuration in the picture above tells us that the electrons are.. Electron Shell Configuration S P D F.
From staff.concord.org
Periodic Table and Bonding Electron Configurations Electron Shell Configuration S P D F The electron configuration in the picture above tells us that the electrons are. The orbital names s, p, d, and f describe electron configuration. These line groups are called sharp, principal, diffuse, and fundamental. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its.. Electron Shell Configuration S P D F.
From ar.inspiredpencil.com
Electron Shells S P D F Electron Shell Configuration S P D F The orbital letters are associated with the angular. The orbital names s, p, d, and f describe electron configuration. These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. To write the electron configuration of an atom, identify the energy level of interest. Electron Shell Configuration S P D F.
From socratic.com
s,p,d,f Orbitals Chemistry Socratic Electron Shell Configuration S P D F The orbital names s, p, d, and f describe electron configuration. The electron configuration in the picture above tells us that the electrons are. These line groups are called sharp, principal, diffuse, and fundamental. The electron configuration of an element shows how electrons are distributed in orbitals— which ones are filled and which ones remain vacant. The electron configuration will. Electron Shell Configuration S P D F.
From pages.swcp.com
Parsing the spdf electron orbital model Electron Shell Configuration S P D F These line groups are called sharp, principal, diffuse, and fundamental. The orbital names s, p, d, and f describe electron configuration. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The electron configuration in the picture above tells us that the electrons are. The electron configuration of an element shows. Electron Shell Configuration S P D F.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Electron Shell Configuration S P D F The electron configuration will tell you what energy level, subshell, and how many electrons occupy that subshell. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different. The orbital names s, p, d, and f describe electron configuration. The electron configuration in the picture above tells us that the electrons are.. Electron Shell Configuration S P D F.