Electrodes In The Electrolysis . Ionic compounds conduct electricity when molten or in. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. reactive metals are extracted from their ores using electrolysis.
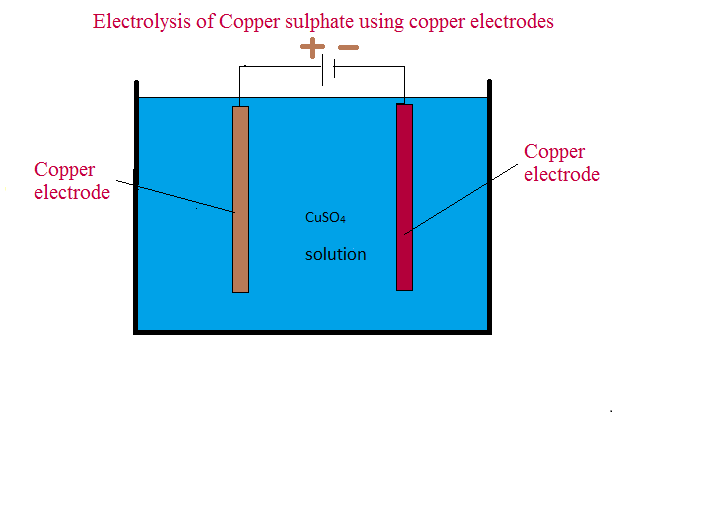
from icsechemistry16.blogspot.com
electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. reactive metals are extracted from their ores using electrolysis. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). Ionic compounds conduct electricity when molten or in.
Electrolysis of copper sulphate using copper electrodes
Electrodes In The Electrolysis electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Electrodes In The Electrolysis in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force. Electrodes In The Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Electrodes In The Electrolysis a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. in a. Electrodes In The Electrolysis.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Electrodes In The Electrolysis in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. electrolysis is carried out in an electrolytic cell consisting of a. Electrodes In The Electrolysis.
From icsechemistry16.blogspot.com
Electrolysis of copper sulphate using copper electrodes Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. Ionic compounds conduct electricity when molten or in. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). electrolysis is carried out in an electrolytic. Electrodes In The Electrolysis.
From hadassah-has-friedman.blogspot.com
Anode and Cathode in Electrolysis HadassahhasFriedman Electrodes In The Electrolysis Ionic compounds conduct electricity when molten or in. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. reactive metals are extracted from their ores using electrolysis. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and. Electrodes In The Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes PtX Hub Electrodes In The Electrolysis electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. Ionic compounds conduct electricity when molten or in. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. a typical electrolytic cell can be made. Electrodes In The Electrolysis.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte. Electrolysis of copper(II) sulfate Electrodes In The Electrolysis Ionic compounds conduct electricity when molten or in. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. electrolysis can occur in electrolytic cells by. Electrodes In The Electrolysis.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Electrodes In The Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. Ionic compounds conduct electricity when molten or in. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). electrolysis is carried out in an electrolytic cell consisting of a positively charged anode. Electrodes In The Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. a typical electrolytic cell can be made as. Electrodes In The Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. . Electrodes In The Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes In The Electrolysis in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. reactive metals are extracted from their ores using electrolysis. electrolysis. Electrodes In The Electrolysis.
From www.alamy.com
Electrolysis process vector illustration. Simple electrolysis process of an electrolyte Stock Electrodes In The Electrolysis electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from their ores using electrolysis. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities,. Electrodes In The Electrolysis.
From www.researchgate.net
Schematic of the membrane electrode assembly (MEA) and catalyst... Download Scientific Diagram Electrodes In The Electrolysis in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). electrolysis. Electrodes In The Electrolysis.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Electrodes In The Electrolysis Ionic compounds conduct electricity when molten or in. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. reactive metals are. Electrodes In The Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Electrodes In The Electrolysis electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from. Electrodes In The Electrolysis.
From www.alamy.com
Electrolysis of copper sulphate solution using copper electrodes Stock Photo Alamy Electrodes In The Electrolysis electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. Ionic compounds conduct. Electrodes In The Electrolysis.
From uen.pressbooks.pub
Electrolysis Introductory Chemistry Electrodes In The Electrolysis in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and. Electrodes In The Electrolysis.
From www.youtube.com
what is the meaning of electrolysis chemistry electrodes electrolysis YouTube Electrodes In The Electrolysis Ionic compounds conduct electricity when molten or in. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. electrolysis is the basis for certain ore. Electrodes In The Electrolysis.
From blog.thepipingmart.com
Why are carbon electrodes used in electrolysis? Electrodes In The Electrolysis in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. Ionic compounds conduct electricity when molten or in. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. electrolysis is the. Electrodes In The Electrolysis.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Electrodes In The Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode. Electrodes In The Electrolysis.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrodes In The Electrolysis in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode. Electrodes In The Electrolysis.
From owlcation.com
Electrolysis The Way of the Future Owlcation Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis. Electrodes In The Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. electrolysis. Electrodes In The Electrolysis.
From wiringfixmashedpatatasz3.z14.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrodes In The Electrolysis electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). reactive metals are extracted from. Electrodes In The Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis. Electrodes In The Electrolysis.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.58C Describe Experiments to Investigate Electrolysis, Using Inert Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. electrolysis is carried out. Electrodes In The Electrolysis.
From www.alamy.com
macrophoto of electrolysis of water in an acid. Two electrodes are placed in an acid and a Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to. Electrodes In The Electrolysis.
From www.snexplores.org
Explainer What is an electrode? Electrodes In The Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). Ionic compounds conduct electricity when molten or in. electrolysis is the basis for certain ore refining processes, the industrial production of many. Electrodes In The Electrolysis.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Electrodes In The Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. reactive metals are extracted from their ores using electrolysis. electrolysis. Electrodes In The Electrolysis.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and. Electrodes In The Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download ID5285503 Electrodes In The Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. in a process called electroplating, a layer of a second metal is deposited on. Electrodes In The Electrolysis.
From mungfali.com
Electrolysis Process Diagram Electrodes In The Electrolysis reactive metals are extracted from their ores using electrolysis. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. Ionic compounds conduct electricity when molten or in. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and. Electrodes In The Electrolysis.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube Electrodes In The Electrolysis in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. electrolysis is carried out in an electrolytic cell consisting of a positively charged anode. Electrodes In The Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes In The Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. in a process called electroplating, a layer of a second metal is deposited on the metal electrode. Electrodes In The Electrolysis.
From www.tes.com
Changes at the electrodes in electrolysis Teaching Resources Electrodes In The Electrolysis a typical electrolytic cell can be made as shown in figure \(\pageindex{1}\). electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of. in a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. Ionic compounds. Electrodes In The Electrolysis.