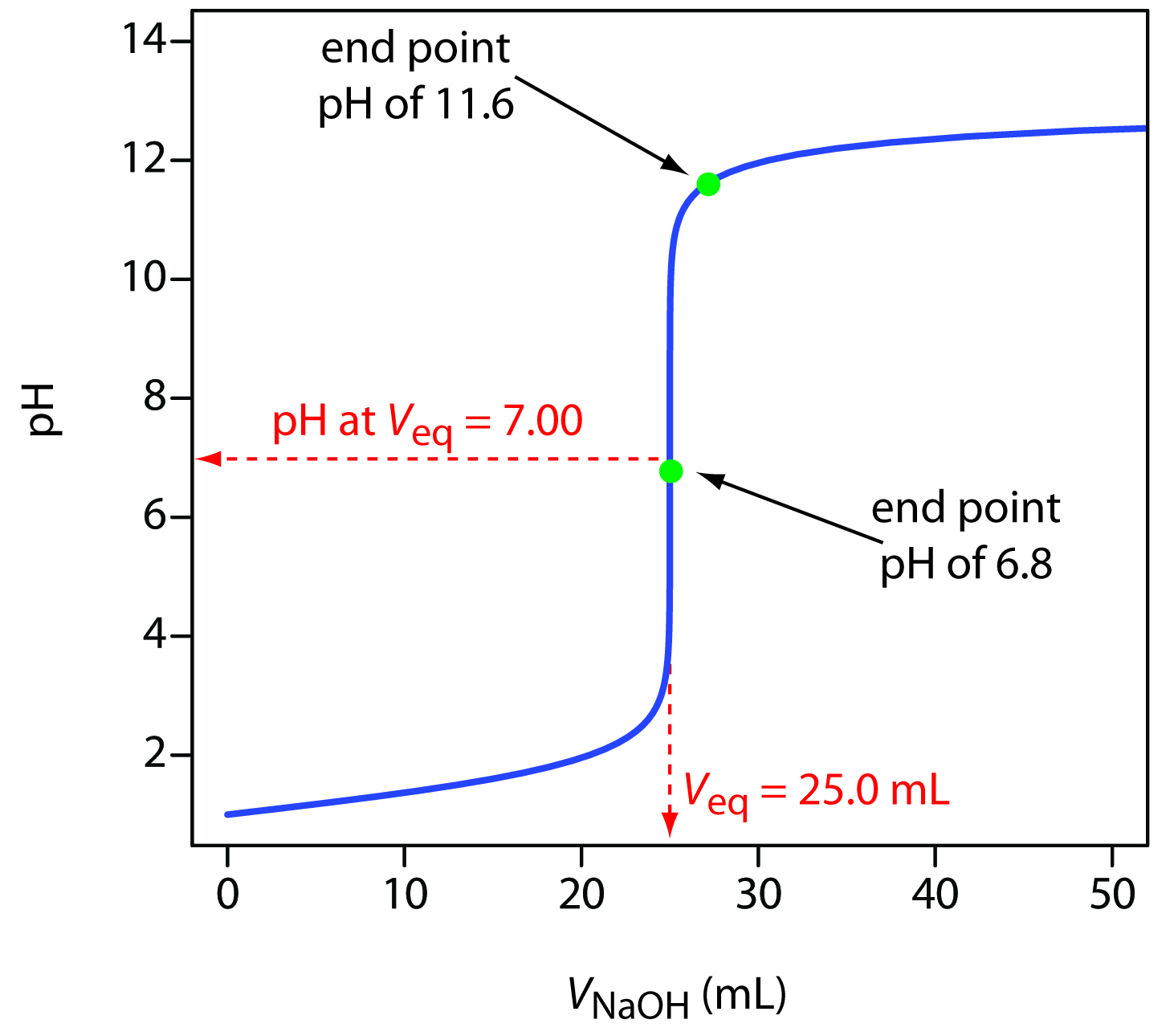
Types Of Titration Curve . As the starting solution is acidic,. Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) how to explain the shape of a titration curve. It provides valuable information about the reaction under. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. The figure below shows two different examples of a. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration curve is a graphical representation of the ph of a solution during a titration. there are four shapes of titration curves that you need to be able to explain. The equivalence point of a titration. there are two basic types of acid base titrations, indicator and potentiometric. You will also learn about equivalence points and. In the examples shown below, alkali is added to an acid. In an indicator based titration you add another. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide.
from chem.libretexts.org
in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. You will also learn about equivalence points and. In an indicator based titration you add another. how to explain the shape of a titration curve. As the starting solution is acidic,. It provides valuable information about the reaction under. In the examples shown below, alkali is added to an acid. The equivalence point of a titration.
9.1 Overview of Titrimetry Chemistry LibreTexts
Types Of Titration Curve As the starting solution is acidic,. In the examples shown below, alkali is added to an acid. a titration curve is a graphical representation of the ph of a solution during a titration. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. In an indicator based titration you add another. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. there are two basic types of acid base titrations, indicator and potentiometric. how to explain the shape of a titration curve. It provides valuable information about the reaction under. You will also learn about equivalence points and. there are four shapes of titration curves that you need to be able to explain. The equivalence point of a titration. As the starting solution is acidic,. The figure below shows two different examples of a. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq)
From saylordotorg.github.io
AcidBase Titrations Types Of Titration Curve The figure below shows two different examples of a. there are two basic types of acid base titrations, indicator and potentiometric. how to explain the shape of a titration curve. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. a titration curve is a graphical representation of. Types Of Titration Curve.
From hicensvanderkruijs.blogspot.com
The Graph Shows The Titration Curves Of A 1M Solution / Consider The Types Of Titration Curve In the examples shown below, alkali is added to an acid. there are two basic types of acid base titrations, indicator and potentiometric. It provides valuable information about the reaction under. The equivalence point of a titration. You will also learn about equivalence points and. As the starting solution is acidic,. the titration curve is a graphical representation. Types Of Titration Curve.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Types Of Titration Curve the titration curve is a graphical representation of the ph or other property changes during a titration experiment. In the examples shown below, alkali is added to an acid. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. In an indicator based titration you add another. It provides valuable. Types Of Titration Curve.
From www.writework.com
Titration of amino acids WriteWork Types Of Titration Curve there are four shapes of titration curves that you need to be able to explain. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. In the examples shown below, alkali. Types Of Titration Curve.
From www.vrogue.co
Types Of Photometric Titration Curves Youtube vrogue.co Types Of Titration Curve As the starting solution is acidic,. there are two basic types of acid base titrations, indicator and potentiometric. there are four shapes of titration curves that you need to be able to explain. The figure below shows two different examples of a. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such. Types Of Titration Curve.
From mungfali.com
Acid Titration Curve Types Of Titration Curve Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) It provides valuable information about the reaction under. As the starting solution is acidic,. In an indicator based titration you add another. You will also learn about equivalence points and. there are four shapes of titration curves that you need to be able to explain. . Types Of Titration Curve.
From 88guru.com
Acid Base Titration What is a Titration Curve? 88guru Types Of Titration Curve As the starting solution is acidic,. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. In an indicator based titration you add another. there are four shapes of titration curves that you need to be able to explain. You will also learn about equivalence points and. Hcl (aq) +. Types Of Titration Curve.
From mavink.com
Strong Acid And Base Titration Curve Types Of Titration Curve As the starting solution is acidic,. a titration curve is a graphical representation of the ph of a solution during a titration. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. The figure below shows two different examples of a. there are four shapes of titration curves that. Types Of Titration Curve.
From dornshuld.chemistry.msstate.edu
5.10 Titration Curves Chemistry Types Of Titration Curve in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. It provides valuable information about the reaction under. there are two basic types of acid base titrations, indicator and potentiometric. how to explain the shape of a titration curve. The figure below shows two different examples of a. As. Types Of Titration Curve.
From ar.inspiredpencil.com
Titration Curve Labeled Types Of Titration Curve It provides valuable information about the reaction under. In the examples shown below, alkali is added to an acid. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. As the starting solution is acidic,. Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) there are. Types Of Titration Curve.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Types Of Titration Curve The equivalence point of a titration. a titration curve is a graphical representation of the ph of a solution during a titration. there are four shapes of titration curves that you need to be able to explain. In the examples shown below, alkali is added to an acid. It provides valuable information about the reaction under. In an. Types Of Titration Curve.
From mavink.com
Titration Diagram Types Of Titration Curve Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) there are two basic types of acid base titrations, indicator and potentiometric. As the starting solution is acidic,. The equivalence point of a titration. It provides valuable information about the reaction under. In the examples shown below, alkali is added to an acid. In an indicator. Types Of Titration Curve.
From www.numerade.com
SOLVED Part D Interpreting Titration Curves Using the graphs shown Types Of Titration Curve how to explain the shape of a titration curve. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. The equivalence point of a titration. there are four shapes of titration curves that you need to be able to explain. a titration curve is a graphical representation of. Types Of Titration Curve.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Types Of Titration Curve there are two basic types of acid base titrations, indicator and potentiometric. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. You will also learn about equivalence points and. The equivalence point of a titration. In an indicator based titration you add another. the titration curve is a. Types Of Titration Curve.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Types Of Titration Curve there are four shapes of titration curves that you need to be able to explain. The equivalence point of a titration. how to explain the shape of a titration curve. a titration curve is a graphical representation of the ph of a solution during a titration. In the examples shown below, alkali is added to an acid.. Types Of Titration Curve.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Types Of Titration Curve The equivalence point of a titration. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. In an indicator based titration you add another. In the examples shown below, alkali is added. Types Of Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Types Of Titration Curve You will also learn about equivalence points and. how to explain the shape of a titration curve. It provides valuable information about the reaction under. there are two basic types of acid base titrations, indicator and potentiometric. The equivalence point of a titration. In the examples shown below, alkali is added to an acid. the titration curve. Types Of Titration Curve.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Types Of Titration Curve the titration curve is a graphical representation of the ph or other property changes during a titration experiment. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. It provides valuable information about the reaction under. Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) . Types Of Titration Curve.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Types Of Titration Curve in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. In the examples. Types Of Titration Curve.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Types Of Titration Curve You will also learn about equivalence points and. In an indicator based titration you add another. The figure below shows two different examples of a. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. The equivalence point of a titration. It provides valuable information about the reaction under. a. Types Of Titration Curve.
From www.youtube.com
Conductometric Titration & Titration Curves // HSC Chemistry YouTube Types Of Titration Curve the titration curve is a graphical representation of the ph or other property changes during a titration experiment. there are four shapes of titration curves that you need to be able to explain. As the starting solution is acidic,. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide.. Types Of Titration Curve.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Types Of Titration Curve there are four shapes of titration curves that you need to be able to explain. In the examples shown below, alkali is added to an acid. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. The figure below shows two different examples of a. Imagine we are titrating a. Types Of Titration Curve.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Types Of Titration Curve The equivalence point of a titration. In the examples shown below, alkali is added to an acid. It provides valuable information about the reaction under. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. there are four shapes of titration curves that you need to be able to explain.. Types Of Titration Curve.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Types Of Titration Curve It provides valuable information about the reaction under. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. In the examples shown below, alkali is added to an acid. You will also learn about equivalence points and. Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) . Types Of Titration Curve.
From mungfali.com
Titration Curve Labeled Types Of Titration Curve Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) You will also learn about equivalence points and. there are four shapes of titration curves that you need to be able to explain. In the examples shown below, alkali is added to an acid. in this tutorial, you will learn about titration curves, titration analysis. Types Of Titration Curve.
From slidetodoc.com
Acid Base Titrations Titration Curve A titration curve Types Of Titration Curve there are two basic types of acid base titrations, indicator and potentiometric. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. In the examples shown below, alkali is added to an acid. there are four shapes of titration curves that you need to be able to explain. You. Types Of Titration Curve.
From revise.im
Buffers and Neutralisation Revise.im Types Of Titration Curve in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. You will also learn about equivalence points and. As the starting solution is acidic,. Hcl (aq) + naoh (aq) ⇾ h 2. Types Of Titration Curve.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Types Of Titration Curve the titration curve is a graphical representation of the ph or other property changes during a titration experiment. In the examples shown below, alkali is added to an acid. there are two basic types of acid base titrations, indicator and potentiometric. how to explain the shape of a titration curve. As the starting solution is acidic,. The. Types Of Titration Curve.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 Types Of Titration Curve the titration curve is a graphical representation of the ph or other property changes during a titration experiment. Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) how to explain the shape of a titration curve. there are two basic types of acid base titrations, indicator and potentiometric. there are four shapes. Types Of Titration Curve.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Types Of Titration Curve In the examples shown below, alkali is added to an acid. a titration curve is a graphical representation of the ph of a solution during a titration. there are two basic types of acid base titrations, indicator and potentiometric. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.. Types Of Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Types Of Titration Curve In the examples shown below, alkali is added to an acid. The figure below shows two different examples of a. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. there are two basic types of acid base titrations, indicator and potentiometric. It provides valuable information about the reaction under.. Types Of Titration Curve.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Types Of Titration Curve Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) there are four shapes of titration curves that you need to be able to explain. In the examples shown below, alkali is added to an acid. a titration curve is a graphical representation of the ph of a solution during a titration. You will also. Types Of Titration Curve.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Types Of Titration Curve a titration curve is a graphical representation of the ph of a solution during a titration. there are four shapes of titration curves that you need to be able to explain. Hcl (aq) + naoh (aq) ⇾ h 2 o (l) + nacl (aq) there are two basic types of acid base titrations, indicator and potentiometric. You. Types Of Titration Curve.
From mavink.com
4 Types Of Titration Curves Types Of Titration Curve Imagine we are titrating a strong acid such as hydrochloric acid against a strong base such as sodium hydroxide. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration curve is a graphical representation of the ph of a solution during a titration. there are two basic. Types Of Titration Curve.
From www.numerade.com
SOLVED Identify each type of titration curve Note that the analyte is Types Of Titration Curve You will also learn about equivalence points and. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. there are two basic types of acid base titrations, indicator and potentiometric. The. Types Of Titration Curve.