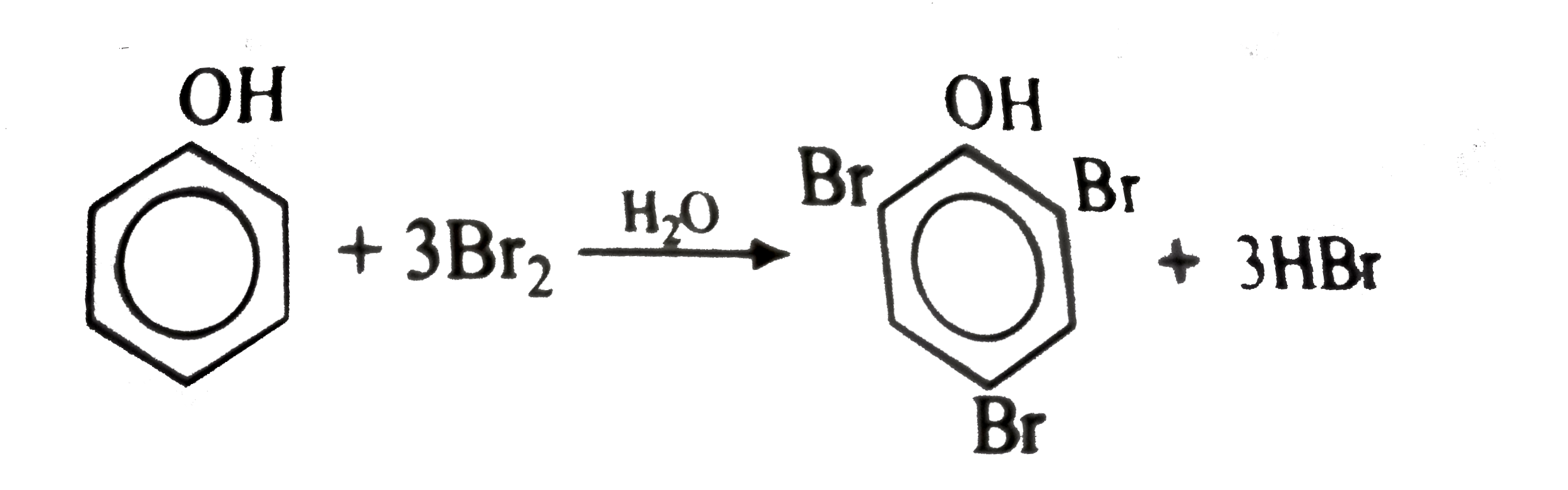Bromine Water Phenol . In phenols, mostly electrophilic substitution. If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic. Write an equation to illustrate the oxidation of a. Bromine water, which contains diatomic bromine molecules, has an orange color. Phenol has a characteristic smell and can act as a germicidal. Phenol is an oxygen substituted organic compound. One way to test for the presence of phenol is to use bromine water. The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). When bromine water is added to a solution. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. It is known to us that if we add bromine water to. Phenol is an insoluble organic compound in water. Complete step by step answer:
from www.doubtnut.com
It is known to us that if we add bromine water to. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. When bromine water is added to a solution. Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). Complete step by step answer: If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic. Write an equation to illustrate the oxidation of a. Phenol has a characteristic smell and can act as a germicidal. Phenol is an oxygen substituted organic compound. In phenols, mostly electrophilic substitution.
When phenol in treated with excess bromine water, it gives
Bromine Water Phenol Phenol is an insoluble organic compound in water. The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. When bromine water is added to a solution. Phenol is an oxygen substituted organic compound. If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic. Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). Complete step by step answer: It is known to us that if we add bromine water to. One way to test for the presence of phenol is to use bromine water. Phenol is an insoluble organic compound in water. Phenol has a characteristic smell and can act as a germicidal. Bromine water, which contains diatomic bromine molecules, has an orange color. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. In phenols, mostly electrophilic substitution. Write an equation to illustrate the oxidation of a.
From www.doubtnut.com
When phenol in treated with excess bromine water, it gives Bromine Water Phenol Phenol is an insoluble organic compound in water. When bromine water is added to a solution. Bromine water, which contains diatomic bromine molecules, has an orange color. It is known to us that if we add bromine water to. Phenol has a characteristic smell and can act as a germicidal. Benzene will undergo bromination only when reacted with pure bromine. Bromine Water Phenol.
From byjus.com
What happens when phenol is treated with bromine? Bromine Water Phenol One way to test for the presence of phenol is to use bromine water. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. It is known to us that if we add bromine water to. Bromine water, which contains diatomic bromine molecules, has an orange color. Write an. Bromine Water Phenol.
From www.doubtnut.com
Explain the action of bromine water on phenol (carbolic acid). Bromine Water Phenol When bromine water is added to a solution. One way to test for the presence of phenol is to use bromine water. It is known to us that if we add bromine water to. Bromine water, which contains diatomic bromine molecules, has an orange color. If bromine water is added to a solution of phenol in water, the bromine water. Bromine Water Phenol.
From www.doubtnut.com
[Telugu] Explain why phenol with bromine water forms 2,4,6tribromophe Bromine Water Phenol Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. Write an equation to illustrate the oxidation of a. One way to test for the presence of phenol is to use bromine water. When bromine water is added to a solution. Complete step by step answer: Bromine water, which. Bromine Water Phenol.
From www.numerade.com
SOLVED solve before 230 How phenol reacts with ? Bromine water (ii Bromine Water Phenol One way to test for the presence of phenol is to use bromine water. In phenols, mostly electrophilic substitution. Write an equation to illustrate the oxidation of a. Phenol is an oxygen substituted organic compound. Phenol has a characteristic smell and can act as a germicidal. When bromine water is added to a solution. Complete step by step answer: The. Bromine Water Phenol.
From www.toppr.com
of what is the action of following on phenol 1) bromine water come HNO3 Bromine Water Phenol Phenol is an oxygen substituted organic compound. It is known to us that if we add bromine water to. Complete step by step answer: Phenol has a characteristic smell and can act as a germicidal. Bromine water, which contains diatomic bromine molecules, has an orange color. Phenol is an insoluble organic compound in water. When bromine water is added to. Bromine Water Phenol.
From www.youtube.com
DEMO PHENOL & BROMINE WATER ELECTROPHILIC SUBSTITUTION (A2 CHEMISTRY Bromine Water Phenol Phenol is an insoluble organic compound in water. Write an equation to illustrate the oxidation of a. Complete step by step answer: Bromine water, which contains diatomic bromine molecules, has an orange color. The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. In phenols, mostly electrophilic substitution. Benzene will undergo bromination only when reacted with. Bromine Water Phenol.
From chemistry.stackexchange.com
organic chemistry Halogenation of Phenol Chemistry Stack Exchange Bromine Water Phenol Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). Phenol is an insoluble organic compound in water. Phenol has a characteristic smell and can act as a germicidal. One way to test for the presence of phenol is to use bromine water. In phenols, mostly electrophilic substitution. It is known. Bromine Water Phenol.
From www.doubtnut.com
When phenol is treated with bromine water, white precipitate is obtain Bromine Water Phenol Complete step by step answer: Bromine water, which contains diatomic bromine molecules, has an orange color. In phenols, mostly electrophilic substitution. The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. Write an equation to illustrate the oxidation of a. Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4. Bromine Water Phenol.
From www.youtube.com
ACTION OF PHENOL WITH BROMINE WATER YouTube Bromine Water Phenol Phenol is an insoluble organic compound in water. Phenol has a characteristic smell and can act as a germicidal. Phenol is an oxygen substituted organic compound. When bromine water is added to a solution. If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of. Bromine Water Phenol.
From www.youtube.com
Reaction of Bromine with Phenol YouTube Bromine Water Phenol It is known to us that if we add bromine water to. If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic. Write an equation to illustrate the oxidation of a. Bromine water, which contains diatomic bromine molecules, has an orange color. The. Bromine Water Phenol.
From www.chemistrystudent.com
Phenol Reactions (ALevel) ChemistryStudent Bromine Water Phenol In phenols, mostly electrophilic substitution. When bromine water is added to a solution. Bromine water, which contains diatomic bromine molecules, has an orange color. Complete step by step answer: It is known to us that if we add bromine water to. Write an equation to illustrate the oxidation of a. Explain why phenols and phenoxide ions are very reactive towards. Bromine Water Phenol.
From byjus.com
Does phenol react with bromine? Bromine Water Phenol Complete step by step answer: Bromine water, which contains diatomic bromine molecules, has an orange color. Phenol is an oxygen substituted organic compound. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4. Bromine Water Phenol.
From www.youtube.com
24) Halogenation of phenol Reaction of phenol with Bromine water and Bromine Water Phenol Bromine water, which contains diatomic bromine molecules, has an orange color. Phenol is an insoluble organic compound in water. In phenols, mostly electrophilic substitution. If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic. Phenol has a characteristic smell and can act as. Bromine Water Phenol.
From www.youtube.com
Bromine water test / Qualitative test of phenol YouTube Bromine Water Phenol Write an equation to illustrate the oxidation of a. Phenol is an insoluble organic compound in water. When bromine water is added to a solution. Complete step by step answer: Phenol is an oxygen substituted organic compound. The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. In phenols, mostly electrophilic substitution. Explain why phenols and. Bromine Water Phenol.
From www.toppr.com
What happens when phenol reacts with bromine water?Write the chemical Bromine Water Phenol One way to test for the presence of phenol is to use bromine water. It is known to us that if we add bromine water to. If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic. Benzene will undergo bromination only when reacted. Bromine Water Phenol.
From www.toppr.com
When phenol is treated with bromine water, while precipitate is Bromine Water Phenol The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. Phenol is an oxygen substituted organic compound. Phenol is an insoluble organic compound in water. Write an equation to illustrate the oxidation of a. Complete. Bromine Water Phenol.
From www.youtube.com
When Phenol reacts with Br + CS2 and Bromine Water Alcohol Phenol Bromine Water Phenol Phenol is an insoluble organic compound in water. Write an equation to illustrate the oxidation of a. Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). Bromine water, which contains diatomic bromine molecules, has an orange color. Phenol has a characteristic smell and can act as a germicidal. In phenols,. Bromine Water Phenol.
From www.doubtnut.com
When phenol in treated with excess bromine water, it gives Bromine Water Phenol Phenol has a characteristic smell and can act as a germicidal. Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. In phenols, mostly electrophilic substitution. One way to test for the presence of phenol is to. Bromine Water Phenol.
From www.youtube.com
bromine with a phenol (derivative) YouTube Bromine Water Phenol If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic. Phenol has a characteristic smell and can act as a germicidal. Phenol is an oxygen substituted organic compound. Write an equation to illustrate the oxidation of a. Complete step by step answer: When. Bromine Water Phenol.
From www.numerade.com
SOLVEDPhenol + bromine water →white precipitate. The product is (a) o Bromine Water Phenol In phenols, mostly electrophilic substitution. When bromine water is added to a solution. Phenol is an oxygen substituted organic compound. Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). Phenol has a characteristic smell and can act as a germicidal. Write an equation to illustrate the oxidation of a. It. Bromine Water Phenol.
From www.youtube.com
Organic Chemistry Experiment Reaction of Phenol with Bromine Water Bromine Water Phenol One way to test for the presence of phenol is to use bromine water. Phenol is an oxygen substituted organic compound. Bromine water, which contains diatomic bromine molecules, has an orange color. It is known to us that if we add bromine water to. Phenol has a characteristic smell and can act as a germicidal. Explain why phenols and phenoxide. Bromine Water Phenol.
From www.doubtnut.com
[Telugu] Explain why phenol with bromine water forms 2,4,6tribromophe Bromine Water Phenol In phenols, mostly electrophilic substitution. Phenol is an oxygen substituted organic compound. The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. Complete step by step answer: Bromine water, which contains diatomic bromine molecules, has. Bromine Water Phenol.
From slidetodoc.com
Phenol Understand the reaction of phenol with bromine Bromine Water Phenol Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). Phenol is an oxygen substituted organic compound. Write an equation to illustrate the oxidation of a. The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. If bromine water is added to a solution of phenol in. Bromine Water Phenol.
From www.youtube.com
Reaction of Phenol With Bromine Alcohols, Phenols and Ethers Bromine Water Phenol Phenol is an insoluble organic compound in water. One way to test for the presence of phenol is to use bromine water. Bromine water, which contains diatomic bromine molecules, has an orange color. Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). It is known to us that if we. Bromine Water Phenol.
From www.doubtnut.com
Phenol reacts with bromine water in the ratio of to give 2,4,6Tribr Bromine Water Phenol Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). Bromine water, which contains diatomic bromine molecules, has an orange color. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. In phenols, mostly electrophilic substitution. The reaction of phenol. Bromine Water Phenol.
From www.youtube.com
Bromine water in Phenol chemistrypractical chemistrysolution YouTube Bromine Water Phenol Phenol is an oxygen substituted organic compound. In phenols, mostly electrophilic substitution. When bromine water is added to a solution. Bromine water, which contains diatomic bromine molecules, has an orange color. Phenol is an insoluble organic compound in water. Write an equation to illustrate the oxidation of a. Complete step by step answer: The reaction of phenol with bromine water. Bromine Water Phenol.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Bromine Water Phenol If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic. Bromine water, which contains diatomic bromine molecules, has an orange color. When bromine water is added to a solution. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and. Bromine Water Phenol.
From www.researchgate.net
Three bromination of phenols; (a) traditional electrophilic Bromine Water Phenol The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. Phenol is an oxygen substituted organic compound. It is known to us that if we add bromine water to. Phenol has a characteristic smell and can act as a germicidal. One way to test for the presence of phenol is to use bromine water. Complete step. Bromine Water Phenol.
From klaehyagl.blob.core.windows.net
Bromine Molecule Formula at Antonio Godines blog Bromine Water Phenol Write an equation to illustrate the oxidation of a. Phenol is an insoluble organic compound in water. When bromine water is added to a solution. It is known to us that if we add bromine water to. In phenols, mostly electrophilic substitution. The reaction of phenol with bromine water results in the formation of polyhalogen derivatives. If bromine water is. Bromine Water Phenol.
From www.sciencephoto.com
Bromination of phenol Stock Image C019/4673 Science Photo Library Bromine Water Phenol It is known to us that if we add bromine water to. Phenol is an oxygen substituted organic compound. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. Complete step by step answer: In phenols, mostly electrophilic substitution. If bromine water is added to a solution of phenol. Bromine Water Phenol.
From www.youtube.com
Reaction of Phenol with Bromine water 2,4,6 tribromo phenol cbse Bromine Water Phenol Phenol is an oxygen substituted organic compound. When bromine water is added to a solution. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence of an anhydrous aluminium. Complete step by step answer: If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a. Bromine Water Phenol.
From www.youtube.com
phenol reacts with Bromine water // phenol into 2,4,6 tribromophenol Bromine Water Phenol Complete step by step answer: Explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see section 16.4 of the textbook). Bromine water, which contains diatomic bromine molecules, has an orange color. Phenol has a characteristic smell and can act as a germicidal. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and. Bromine Water Phenol.
From www.chemistrystudent.com
Organic Quick Notes (revision) for A2Level ChemistryStudent Bromine Water Phenol Phenol has a characteristic smell and can act as a germicidal. Phenol is an oxygen substituted organic compound. Phenol is an insoluble organic compound in water. Complete step by step answer: It is known to us that if we add bromine water to. Benzene will undergo bromination only when reacted with pure bromine (not a solution) and in the presence. Bromine Water Phenol.
From www.nagwa.com
Question Video Describing the Reaction of Phenol and Alkenes with Bromine Water Phenol Phenol is an oxygen substituted organic compound. When bromine water is added to a solution. Bromine water, which contains diatomic bromine molecules, has an orange color. If bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic. Complete step by step answer: Write an. Bromine Water Phenol.