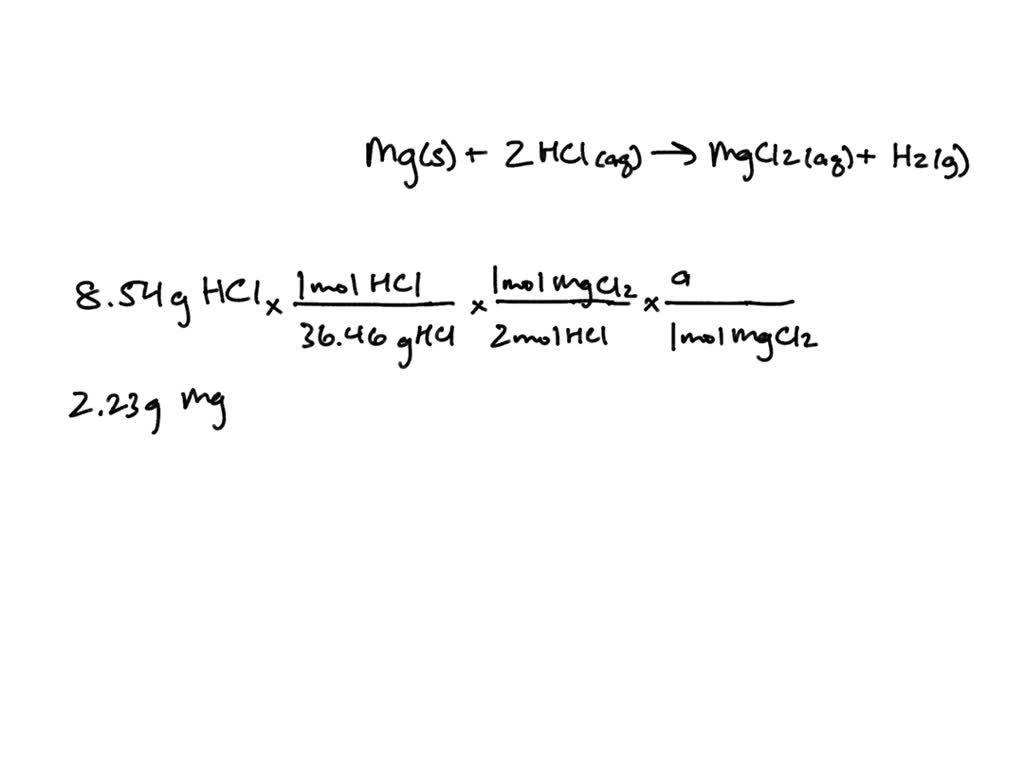
Do Bases React With Mg . Hydrochloric acid + magnesium →. Acid + metal → salt + hydrogen. 2hno 3 + mgo → mg (no 3) 2 + h 2 o. Although acids and bases have their own unique chemistries, the acid and base. Acids react with metals to produce a salt and hydrogen. 2hcl (aq) + mg (s) → mgcl. Some bases can also react with metals to form a gaseous product. Acid + base → salt + water. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Hydrochloric acid + magnesium → magnesium chloride + hydrogen. For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. Acid + metal → salt + hydrogen. Nitric acid + magnesium oxide → magnesium nitrate + water. The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. When acids react with a base, a salt and water are made.
from www.vrogue.co
Acid + metal → salt + hydrogen. Some bases can also react with metals to form a gaseous product. The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. Acid + base → salt + water. Hydrochloric acid + magnesium → magnesium chloride + hydrogen. Acid + metal → salt + hydrogen. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): When acids react with a base, a salt and water are made. For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. 2hno 3 + mgo → mg (no 3) 2 + h 2 o.
Solved A Piece Of Magnesium Reacts With An Aqueous So vrogue.co
Do Bases React With Mg Hydrochloric acid + magnesium → magnesium chloride + hydrogen. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Acids react with metals to produce a salt and hydrogen. Hydrochloric acid + magnesium → magnesium chloride + hydrogen. For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. Although acids and bases have their own unique chemistries, the acid and base. Acid + base → salt + water. 2hno 3 + mgo → mg (no 3) 2 + h 2 o. When acids react with a base, a salt and water are made. The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. Acid + metal → salt + hydrogen. Hydrochloric acid + magnesium →. Some bases can also react with metals to form a gaseous product. 2hcl (aq) + mg (s) → mgcl. Acid + metal → salt + hydrogen. Nitric acid + magnesium oxide → magnesium nitrate + water.
From www.youtube.com
Why does Magnesium react with Water? YouTube Do Bases React With Mg Acid + base → salt + water. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Some bases can also react with metals to form a gaseous product. Acid + metal → salt + hydrogen. Nitric acid + magnesium oxide → magnesium nitrate + water. Acid + metal → salt +. Do Bases React With Mg.
From mavink.com
Magnesium And Hcl Reaction Do Bases React With Mg Although acids and bases have their own unique chemistries, the acid and base. For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. Some bases can also react with metals to form a gaseous product. Hydrochloric acid + magnesium →. Acid + metal → salt + hydrogen. 2hcl (aq) + mg (s) → mgcl. Acid + base →. Do Bases React With Mg.
From www.chemistrysteps.com
The Grignard Reaction Mechanism Chemistry Steps Do Bases React With Mg Acids react with metals to produce a salt and hydrogen. For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. Hydrochloric acid + magnesium → magnesium chloride + hydrogen. Although acids and bases have their own unique chemistries, the acid and base. Some bases can also react with metals to form a gaseous product. Hydrochloric acid + magnesium. Do Bases React With Mg.
From educationgrafts.z21.web.core.windows.net
What Do Bases React With Do Bases React With Mg Acids react with metals to produce a salt and hydrogen. 2hno 3 + mgo → mg (no 3) 2 + h 2 o. When acids react with a base, a salt and water are made. Acid + base → salt + water. Although acids and bases have their own unique chemistries, the acid and base. 2hcl (aq) + mg (s). Do Bases React With Mg.
From www.sliderbase.com
Properties of Acids Bases Presentation Chemistry Do Bases React With Mg Although acids and bases have their own unique chemistries, the acid and base. Hydrochloric acid + magnesium →. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Some bases can also react with metals to form a gaseous product. Acid + metal → salt + hydrogen. Acids react with metals to. Do Bases React With Mg.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts Do Bases React With Mg Although acids and bases have their own unique chemistries, the acid and base. Some bases can also react with metals to form a gaseous product. Acid + metal → salt + hydrogen. Acids react with metals to produce a salt and hydrogen. Acid + base → salt + water. The reaction that happens when an acid, such as \(\ce{hcl}\), is. Do Bases React With Mg.
From www.youtube.com
How do Acids and Bases React with Metals ! अम्ल की धातु से क्रिया Do Bases React With Mg For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. Some bases can also react with metals to form a gaseous product. The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. Acid + metal → salt + hydrogen. Hydrochloric acid + magnesium → magnesium. Do Bases React With Mg.
From educationgrafts.z21.web.core.windows.net
What Do Bases React With Do Bases React With Mg Although acids and bases have their own unique chemistries, the acid and base. Some bases can also react with metals to form a gaseous product. Nitric acid + magnesium oxide → magnesium nitrate + water. Acids react with metals to produce a salt and hydrogen. Hydrochloric acid + magnesium →. The reaction that happens when an acid, such as \(\ce{hcl}\),. Do Bases React With Mg.
From www.slideserve.com
PPT Acids react with zinc, magnesium, or aluminum and form hydrogen Do Bases React With Mg Acid + metal → salt + hydrogen. Some bases can also react with metals to form a gaseous product. 2hno 3 + mgo → mg (no 3) 2 + h 2 o. Acid + metal → salt + hydrogen. For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. Nitric acid + magnesium oxide → magnesium nitrate +. Do Bases React With Mg.
From www.teachoo.com
How do Acids and Bases react with Metals? [with Examples] Teachoo Do Bases React With Mg The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. Some bases can also react with metals to form a gaseous product. 2hcl (aq) + mg (s) → mgcl. Acid + metal → salt + hydrogen. For instance, strong bases, such as sodium hydroxide and potassium hydroxide,. Do Bases React With Mg.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Do Bases React With Mg Acids react with metals to produce a salt and hydrogen. 2hno 3 + mgo → mg (no 3) 2 + h 2 o. Although acids and bases have their own unique chemistries, the acid and base. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Nitric acid + magnesium oxide →. Do Bases React With Mg.
From www.yaclass.in
How do bases react with metals — lesson. Science CBSE, Class 10. Do Bases React With Mg Acid + metal → salt + hydrogen. Although acids and bases have their own unique chemistries, the acid and base. Some bases can also react with metals to form a gaseous product. Acids react with metals to produce a salt and hydrogen. Hydrochloric acid + magnesium → magnesium chloride + hydrogen. Nitric acid + magnesium oxide → magnesium nitrate +. Do Bases React With Mg.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses Do Bases React With Mg The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. Hydrochloric acid + magnesium →. When acids react with a base, a salt and water are made. 2hcl (aq) + mg (s) → mgcl. Although acids and bases have their own unique chemistries, the acid and base.. Do Bases React With Mg.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Do Bases React With Mg The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Nitric acid + magnesium oxide → magnesium nitrate + water. Acids react with metals to produce a salt and hydrogen. Hydrochloric acid + magnesium → magnesium chloride + hydrogen. When acids react with a base, a salt and water are made. Hydrochloric. Do Bases React With Mg.
From www.chemicals.co.uk
What is the Difference Between an Acid and a Base? The Chemistry Blog Do Bases React With Mg Nitric acid + magnesium oxide → magnesium nitrate + water. Acid + metal → salt + hydrogen. Hydrochloric acid + magnesium → magnesium chloride + hydrogen. 2hno 3 + mgo → mg (no 3) 2 + h 2 o. When acids react with a base, a salt and water are made. 2hcl (aq) + mg (s) → mgcl. Acid +. Do Bases React With Mg.
From slideplayer.com
Classifying Chemical ____________ ppt download Do Bases React With Mg Acids react with metals to produce a salt and hydrogen. When acids react with a base, a salt and water are made. The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. 2hcl (aq) + mg (s) → mgcl. Nitric acid + magnesium oxide → magnesium nitrate. Do Bases React With Mg.
From slideplayer.com
Acids and Bases. ppt download Do Bases React With Mg The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. 2hcl (aq) + mg (s) → mgcl. Acid + metal → salt + hydrogen. The reaction that happens when an acid, such as \(\ce{hcl}\), is. Do Bases React With Mg.
From www.youtube.com
How do Acids and Bases React with Metals in Hindi for Class 10 YouTube Do Bases React With Mg Hydrochloric acid + magnesium →. Acids react with metals to produce a salt and hydrogen. Some bases can also react with metals to form a gaseous product. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Hydrochloric acid + magnesium → magnesium chloride + hydrogen. 2hcl (aq) + mg (s) →. Do Bases React With Mg.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Do Bases React With Mg Hydrochloric acid + magnesium →. Some bases can also react with metals to form a gaseous product. Acid + metal → salt + hydrogen. Nitric acid + magnesium oxide → magnesium nitrate + water. 2hcl (aq) + mg (s) → mgcl. Acid + metal → salt + hydrogen. 2hno 3 + mgo → mg (no 3) 2 + h 2. Do Bases React With Mg.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Do Bases React With Mg Some bases can also react with metals to form a gaseous product. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Nitric acid + magnesium oxide → magnesium nitrate + water. 2hcl (aq) + mg (s) → mgcl. Hydrochloric acid + magnesium →. 2hno 3 + mgo → mg (no 3). Do Bases React With Mg.
From educationgrafts.z21.web.core.windows.net
What Do Bases React With Do Bases React With Mg Hydrochloric acid + magnesium →. Acid + base → salt + water. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Hydrochloric acid + magnesium → magnesium chloride + hydrogen. The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of. Do Bases React With Mg.
From www.numerade.com
SOLVED If we react benzoic acid with CH3MgBr we obtain to. acid base Do Bases React With Mg Some bases can also react with metals to form a gaseous product. Acid + metal → salt + hydrogen. For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. Acids react with metals to produce a salt and hydrogen. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Nitric. Do Bases React With Mg.
From www.numerade.com
SOLVEDWhich acid and base react to give an aqueous solution of Do Bases React With Mg Acid + base → salt + water. Acid + metal → salt + hydrogen. Some bases can also react with metals to form a gaseous product. The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. Hydrochloric acid + magnesium →. 2hcl (aq) + mg (s) →. Do Bases React With Mg.
From www.toppr.com
How do acids and bases react with metals? Do Bases React With Mg The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. Acid + base → salt + water. 2hno 3 + mgo → mg (no 3) 2 + h 2 o. Although acids and bases have their own unique chemistries, the acid and base. Hydrochloric acid + magnesium. Do Bases React With Mg.
From www.youtube.com
How do Acids and Bases React with each other // Activity 2.6 Class 10 Do Bases React With Mg Nitric acid + magnesium oxide → magnesium nitrate + water. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): When acids react with a base, a salt and water are made. Acid + metal → salt + hydrogen. Acid + metal → salt + hydrogen. Some bases can also react with. Do Bases React With Mg.
From www.teachoo.com
Reaction of Metals and Nonmetals With Base Teachoo Concepts Do Bases React With Mg 2hno 3 + mgo → mg (no 3) 2 + h 2 o. Acid + base → salt + water. Acids react with metals to produce a salt and hydrogen. Nitric acid + magnesium oxide → magnesium nitrate + water. The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care. Do Bases React With Mg.
From www.youtube.com
Heat of Formation of MgO YouTube Do Bases React With Mg The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Although acids and bases have their own unique chemistries, the acid and base. Acid + metal → salt + hydrogen. Acid + metal → salt + hydrogen. 2hno 3 + mgo → mg (no 3) 2 + h 2 o. The base. Do Bases React With Mg.
From www.showme.com
Mg and methanoic acid Science, Chemicalreactions, Chemistry Do Bases React With Mg For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Although acids and bases have their own unique chemistries, the acid and base. Acid + metal → salt + hydrogen. Hydrochloric acid + magnesium → magnesium chloride + hydrogen. The. Do Bases React With Mg.
From www.chemistrystudent.com
Group 2 Metal Reactions (ALevel) ChemistryStudent Do Bases React With Mg The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Hydrochloric acid + magnesium → magnesium chloride + hydrogen. 2hcl (aq) + mg (s) → mgcl. Acid + base → salt + water. Some bases can also react with metals to form a gaseous product. Acid + metal → salt + hydrogen.. Do Bases React With Mg.
From www.youtube.com
Identify the gas evolved when Magnesium reacts with very dilute nitric Do Bases React With Mg For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. 2hcl (aq) + mg (s) → mgcl. Some bases can also react with metals to form a gaseous product. Nitric acid + magnesium oxide → magnesium nitrate + water. Acid + metal → salt + hydrogen. Although acids and bases have their own unique chemistries, the acid and. Do Bases React With Mg.
From www.nagwa.com
Question Video Ordering the Reactions of Magnesium Metal with Varying Do Bases React With Mg 2hcl (aq) + mg (s) → mgcl. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Acid + metal → salt + hydrogen. For instance, strong bases, such as sodium hydroxide and potassium hydroxide, react. Acids react with metals to produce a salt and hydrogen. Acid + base → salt +. Do Bases React With Mg.
From www.vrogue.co
Solved A Piece Of Magnesium Reacts With An Aqueous So vrogue.co Do Bases React With Mg Acids react with metals to produce a salt and hydrogen. Some bases can also react with metals to form a gaseous product. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Although acids and bases have their own unique chemistries, the acid and base. 2hcl (aq) + mg (s) → mgcl.. Do Bases React With Mg.
From www.youtube.com
How bases react with metals CBSE Class 10 Chemistry Notes YouTube Do Bases React With Mg Hydrochloric acid + magnesium →. Hydrochloric acid + magnesium → magnesium chloride + hydrogen. The base in the antacid will react with the \ (\ce {hcl}\) in the stomach and neutralize it, taking care of that unpleasant. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): When acids react with a. Do Bases React With Mg.
From www.youtube.com
Acids Bases and Salts Lesson 09 How do Acids and Bases react with Do Bases React With Mg Hydrochloric acid + magnesium → magnesium chloride + hydrogen. Acid + metal → salt + hydrogen. Acid + base → salt + water. Although acids and bases have their own unique chemistries, the acid and base. 2hno 3 + mgo → mg (no 3) 2 + h 2 o. Acids react with metals to produce a salt and hydrogen. 2hcl. Do Bases React With Mg.
From www.youtube.com
How do acids and bases react with metals. Class X Chemistry YouTube Do Bases React With Mg Acid + metal → salt + hydrogen. When acids react with a base, a salt and water are made. The reaction that happens when an acid, such as \(\ce{hcl}\), is mixed with a base, such as \(\ce{naoh}\): Hydrochloric acid + magnesium → magnesium chloride + hydrogen. Some bases can also react with metals to form a gaseous product. 2hcl (aq). Do Bases React With Mg.