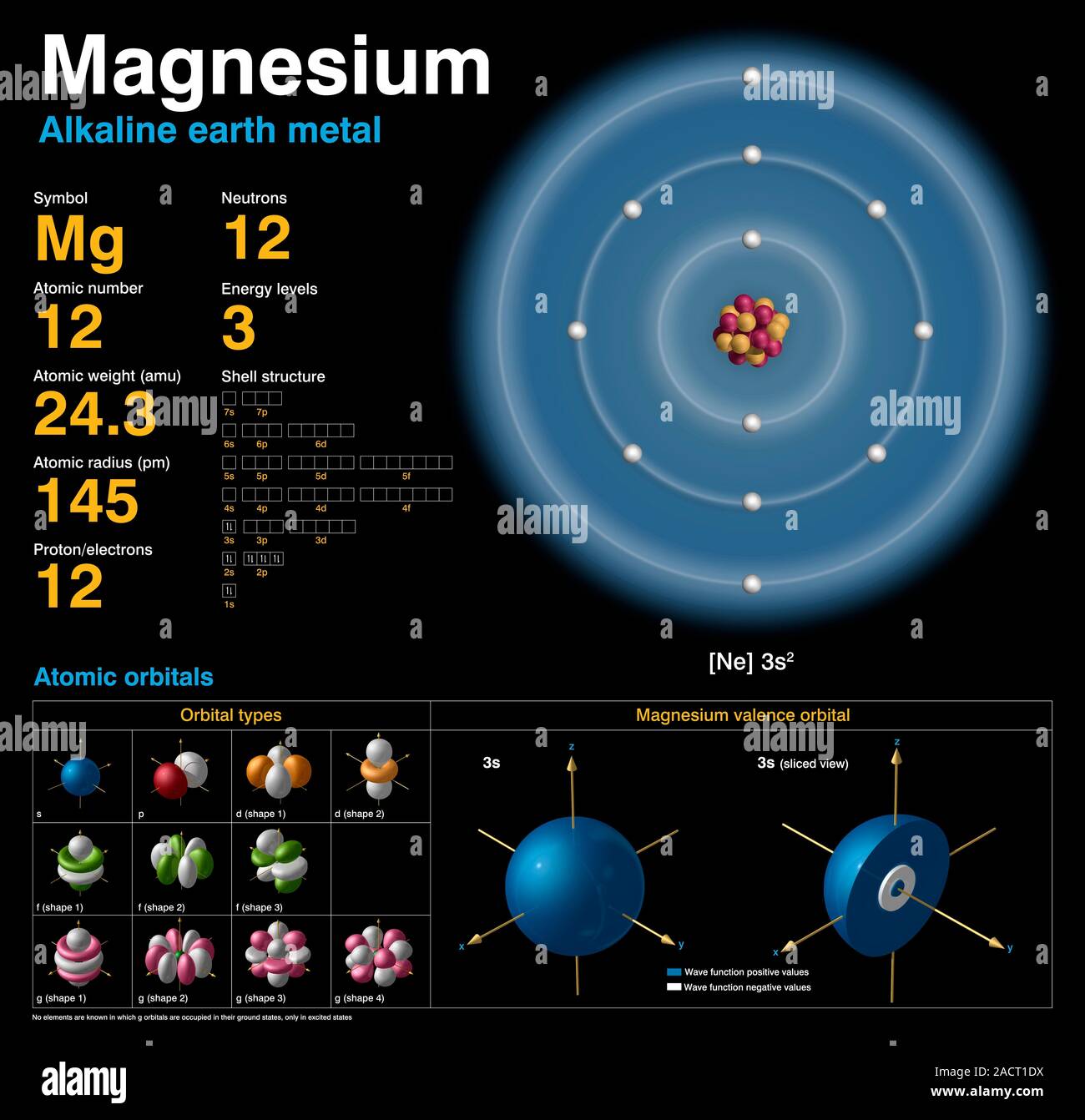
Electron Configuration Of Magnesium Long Form . The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Magnesium has 12 electrons arranged in three energy levels. Electron configuration chart of all elements is mentioned in the table below. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The shorthand electron configuration (or noble gas configuration) as well as. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Magnesium has two valence electrons in the 3s orbital.
from www.alamy.com
Magnesium has two valence electrons in the 3s orbital. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration chart of all elements is mentioned in the table below. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family.
Magnesium (Mg). Diagram of the nuclear composition, electron
Electron Configuration Of Magnesium Long Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. Electron configuration chart of all elements is mentioned in the table below.
From ar.inspiredpencil.com
Magnesium Electron Configuration Notation Electron Configuration Of Magnesium Long Form The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. Electron configuration chart of all elements is mentioned in the table below. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The. Electron Configuration Of Magnesium Long Form.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium Long Form This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. In order to write the mg electron configuration we. Electron Configuration Of Magnesium Long Form.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron Electron Configuration Of Magnesium Long Form Magnesium has 12 electrons arranged in three energy levels. Electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the mg electron configuration we first need to know the number of electrons for the mg. Electron Configuration Of Magnesium Long Form.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Electron Configuration Of Magnesium Long Form The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the. Electron Configuration Of Magnesium Long Form.
From ar.inspiredpencil.com
Magnesium Atom Diagram Electron Configuration Of Magnesium Long Form The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of. Electron Configuration Of Magnesium Long Form.
From www.thesciencehive.co.uk
Atomic Structure and Electron Configuration (AQA) — the science hive Electron Configuration Of Magnesium Long Form Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. This electron configuration calculator will instantly show you the distribution. Electron Configuration Of Magnesium Long Form.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Electron Configuration Of Magnesium Long Form Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The shorthand electron configuration (or noble gas configuration) as well as. The alkaline earth metal magnesium (atomic number 12), with its 12. Electron Configuration Of Magnesium Long Form.
From www.toppr.com
Write the electron dot structure of magnesium and oxygen. Electron Configuration Of Magnesium Long Form In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. Magnesium has 12. Electron Configuration Of Magnesium Long Form.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Magnesium Long Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. In order to write the mg electron configuration we first need. Electron Configuration Of Magnesium Long Form.
From chem.libretexts.org
6.9 Electron Configurations & the Periodic Table Chemistry LibreTexts Electron Configuration Of Magnesium Long Form The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s². Electron Configuration Of Magnesium Long Form.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Magnesium Long Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. Magnesium has two valence electrons in the 3s orbital. Magnesium has 12 electrons arranged in three energy levels. In order to write the mg electron configuration we first. Electron Configuration Of Magnesium Long Form.
From ar.inspiredpencil.com
Magnesium Atom Structure Electron Configuration Of Magnesium Long Form Magnesium has two valence electrons in the 3s orbital. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The shorthand electron configuration (or noble gas configuration) as well as. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration,. Electron Configuration Of Magnesium Long Form.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Magnesium Long Form This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. In order to write the mg electron configuration we first need to know the number of electrons for. Electron Configuration Of Magnesium Long Form.
From www.newtondesk.com
magnesium electron configuration Newton Desk Electron Configuration Of Magnesium Long Form All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The shorthand electron configuration (or noble gas configuration) as well as. The alkaline earth metal magnesium (atomic number. Electron Configuration Of Magnesium Long Form.
From www.youtube.com
Electronic configuration for Magnesium (Mg) spdf Trick Chemistry Electron Configuration Of Magnesium Long Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration,. Electron Configuration Of Magnesium Long Form.
From www.numerade.com
SOLVED a) Write down the electronic configuration of magnesium. b Electron Configuration Of Magnesium Long Form Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Magnesium has 12 electrons arranged in three energy levels. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in. Electron Configuration Of Magnesium Long Form.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Electron Configuration Of Magnesium Long Form This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The shorthand electron configuration (or noble gas configuration) as well as. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of magnesium. Electron Configuration Of Magnesium Long Form.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium Long Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. All elements of group 2 have the same configuration of an electron in the outer electron. Electron Configuration Of Magnesium Long Form.
From www.webelements.com
Elements Periodic Table » Magnesium » properties of free atoms Electron Configuration Of Magnesium Long Form Magnesium has 12 electrons arranged in three energy levels. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of. Electron Configuration Of Magnesium Long Form.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Electron Configuration Of Magnesium Long Form Magnesium has 12 electrons arranged in three energy levels. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. Magnesium has two valence electrons in the 3s orbital. Electron configuration chart of all elements is mentioned in the table below. All elements of group 2 have the same. Electron Configuration Of Magnesium Long Form.
From www.youtube.com
Electron Configuration of Magnesium Mg Lesson YouTube Electron Configuration Of Magnesium Long Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in the 3s orbital. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Electron Configuration Of Magnesium Long Form.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Electron Configuration Of Magnesium Long Form All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium has two valence electrons in the 3s orbital. Magnesium has 12. Electron Configuration Of Magnesium Long Form.
From www.pinterest.se
GensonScience Magnesium Atom model project, Atom model, Atoms and Electron Configuration Of Magnesium Long Form All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Magnesium has 12 electrons arranged in three energy levels. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. Electron configuration chart of all elements. Electron Configuration Of Magnesium Long Form.
From ar.inspiredpencil.com
Electron Configuration Magnesium Electron Configuration Of Magnesium Long Form Electron configuration chart of all elements is mentioned in the table below. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium has two valence electrons in the 3s orbital. All elements of group 2 have the same configuration of an electron in the. Electron Configuration Of Magnesium Long Form.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Electron Configuration Of Magnesium Long Form The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s². Electron Configuration Of Magnesium Long Form.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Electron Configuration Of Magnesium Long Form Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble gas configuration) as well as. Magnesium has two valence electrons in the 3s orbital. This electron configuration calculator will instantly show you the distribution of. Electron Configuration Of Magnesium Long Form.
From sciencenotes.org
List of Electron Configurations of Elements Electron Configuration Of Magnesium Long Form Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. In order to write the mg electron configuration we first need to know the number of electrons for. Electron Configuration Of Magnesium Long Form.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Electron Configuration Of Magnesium Long Form Electron configuration chart of all elements is mentioned in the table below. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family. Magnesium has 12 electrons arranged in three energy levels. All elements of group 2 have the same configuration of an electron in the outer electron shell. Electron Configuration Of Magnesium Long Form.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Magnesium Long Form The shorthand electron configuration (or noble gas configuration) as well as. Magnesium has two valence electrons in the 3s orbital. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The alkaline earth metal magnesium (atomic number 12), with its 12. Electron Configuration Of Magnesium Long Form.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Magnesium Long Form The shorthand electron configuration (or noble gas configuration) as well as. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in the 3s orbital. The alkaline earth. Electron Configuration Of Magnesium Long Form.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Electron Configuration Of Magnesium Long Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below.. Electron Configuration Of Magnesium Long Form.
From uzabytaodi.blogspot.com
41 orbital diagram of magnesium Wiring Diagrams Manual Electron Configuration Of Magnesium Long Form This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium has 12 electrons arranged in three energy levels. The shorthand electron configuration (or noble gas. Electron Configuration Of Magnesium Long Form.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Electron Configuration Of Magnesium Long Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Electron. Electron Configuration Of Magnesium Long Form.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Electron Configuration Of Magnesium Long Form In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Magnesium has 12 electrons arranged in three energy levels. The alkaline earth. Electron Configuration Of Magnesium Long Form.
From www.youtube.com
Magnesium Ground State Electron Configuration YouTube Electron Configuration Of Magnesium Long Form Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium has two valence electrons in the 3s orbital. The electron configuration. Electron Configuration Of Magnesium Long Form.