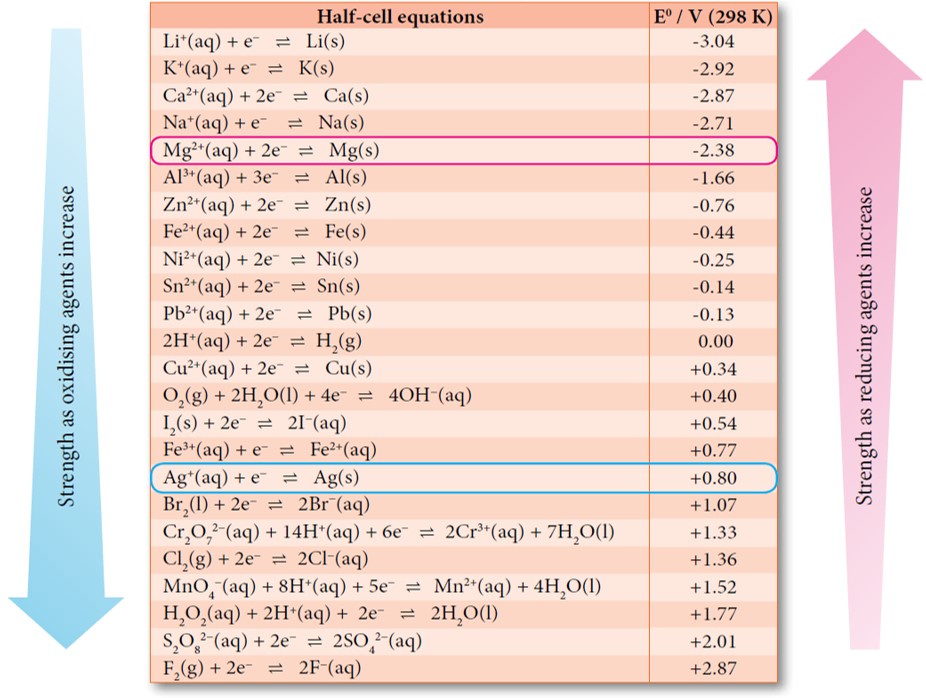
Standard Cell Potential For Zinc And Copper . In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the same. The cell potential difference is shown with the polarity of the right hand electrode; Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e. Since zinc is more reactive than copper, zinc loses electrons more readily to. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. The cell convention for the zinc and copper cell would be; Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. To find the difference of. And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. The 2 ionic half equations of the cell are given below.
from pandai.me
Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e. The cell convention for the zinc and copper cell would be; Since zinc is more reactive than copper, zinc loses electrons more readily to. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The 2 ionic half equations of the cell are given below. In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the same. To find the difference of. And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get.
Standard Electrode Potential
Standard Cell Potential For Zinc And Copper Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e. In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the same. And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The 2 ionic half equations of the cell are given below. The cell potential difference is shown with the polarity of the right hand electrode; To find the difference of. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. Since zinc is more reactive than copper, zinc loses electrons more readily to. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The cell convention for the zinc and copper cell would be; Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e.
From saylordotorg.github.io
Standard Potentials Standard Cell Potential For Zinc And Copper The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. The cell potential difference is shown with the polarity of the right hand. Standard Cell Potential For Zinc And Copper.
From www.numerade.com
SOLVED Calculate the cell potential for a cell with copper as the Standard Cell Potential For Zinc And Copper Since zinc is more reactive than copper, zinc loses electrons more readily to. The cell convention for the zinc and copper cell would be; To find the difference of. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. The cell potential difference is shown with the polarity of the right hand. Standard Cell Potential For Zinc And Copper.
From www.chegg.com
Solved I. Reduction Potential And Reaction Spontaneity A.... Standard Cell Potential For Zinc And Copper The cell convention for the zinc and copper cell would be; And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. In 1780, luigi galvani discovered that when two different. Standard Cell Potential For Zinc And Copper.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential For Zinc And Copper The 2 ionic half equations of the cell are given below. And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. Since zinc is more reactive than copper, zinc loses electrons more readily to. The cell convention for the zinc and copper cell would be; Since the tabulated standard electrode potentials are reduction potentials,. Standard Cell Potential For Zinc And Copper.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors Standard Cell Potential For Zinc And Copper The cell convention for the zinc and copper cell would be; The 2 ionic half equations of the cell are given below. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then. Standard Cell Potential For Zinc And Copper.
From pandai.me
Standard Electrode Potential Standard Cell Potential For Zinc And Copper In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the same. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The cell convention for the zinc and copper cell would be; The cell potential difference is. Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Standard Cell Potential For Zinc And Copper The cell convention for the zinc and copper cell would be; 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative. Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Standard Cell Potential For Zinc And Copper 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. Since zinc is more reactive than copper, zinc loses electrons more readily to. Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e. The cell potential difference is shown with the polarity of the right hand electrode; The standard cell potential (\(e^o_{cell}\)) is. Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID165903 Standard Cell Potential For Zinc And Copper Since zinc is more reactive than copper, zinc loses electrons more readily to. The cell potential difference is shown with the polarity of the right hand electrode; The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. To find. Standard Cell Potential For Zinc And Copper.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Standard Cell Potential For Zinc And Copper And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. The 2 ionic half equations of the cell are given below. The cell convention for the zinc and copper cell would be; The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other. Standard Cell Potential For Zinc And Copper.
From www.chegg.com
Solved 5. What is the standard cell potential (E cel) for Standard Cell Potential For Zinc And Copper To find the difference of. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The cell potential difference is shown with the polarity of the right hand electrode; The cell convention for the zinc and copper cell would be; The potential of the cell under. Standard Cell Potential For Zinc And Copper.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Standard Cell Potential For Zinc And Copper And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e. Since zinc is more reactive than copper, zinc loses electrons more readily to. In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched. Standard Cell Potential For Zinc And Copper.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Standard Cell Potential For Zinc And Copper To find the difference of. The 2 ionic half equations of the cell are given below. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both. Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT Electrochemical Equilibria PowerPoint Presentation, free download Standard Cell Potential For Zinc And Copper Since zinc is more reactive than copper, zinc loses electrons more readily to. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of.. Standard Cell Potential For Zinc And Copper.
From www.chegg.com
Solved 1. Using the theoretical standard reduction potential Standard Cell Potential For Zinc And Copper Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the same. The standard cell potential (\(e^o_{cell}\)) is the difference of the two. Standard Cell Potential For Zinc And Copper.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Cell Potential For Zinc And Copper The cell potential difference is shown with the polarity of the right hand electrode; 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The 2 ionic half. Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Standard Cell Potential For Zinc And Copper Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. The cell potential difference is shown with the polarity of the right hand electrode; Zn (s)∣zn 2+ (aq). Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Standard Cell Potential For Zinc And Copper To find the difference of. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Since zinc is more reactive than copper, zinc loses electrons more readily to. The cell convention for the zinc and copper cell would be; The potential of the cell under standard conditions (1 m for solutions,. Standard Cell Potential For Zinc And Copper.
From www.toppr.com
The standard reduction potentials of Zn^2 + Zn and Cu^2 + Cu are Standard Cell Potential For Zinc And Copper To find the difference of. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The 2 ionic half equations of the cell are given below. Since zinc is more reactive than copper, zinc loses electrons more readily to.. Standard Cell Potential For Zinc And Copper.
From byjus.com
Variation of Cell Potential in ZnCu Cell Chemistry Practicals Class Standard Cell Potential For Zinc And Copper And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. Since zinc is more reactive than copper, zinc loses electrons more readily to. Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e. The cell convention for the zinc and copper cell would be; Since the tabulated standard electrode potentials are reduction potentials, the one. Standard Cell Potential For Zinc And Copper.
From www.numerade.com
SOLVEDeant 3 Explainand show YouL work questions Use the following Standard Cell Potential For Zinc And Copper Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. Since zinc is more reactive than copper, zinc loses electrons more readily to. The 2 ionic half equations. Standard Cell Potential For Zinc And Copper.
From www.youtube.com
Determination of Standard Electrode potential of 'Zn and Cu Standard Cell Potential For Zinc And Copper Since zinc is more reactive than copper, zinc loses electrons more readily to. The 2 ionic half equations of the cell are given below. The cell potential difference is shown with the polarity of the right hand electrode; Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e. To find the difference of. The cell convention for the zinc and copper. Standard Cell Potential For Zinc And Copper.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Standard Cell Potential For Zinc And Copper Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. To find the difference of. The 2 ionic half equations of the cell are given below. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Since. Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential For Zinc And Copper The 2 ionic half equations of the cell are given below. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The cell convention for the zinc and copper cell would be; Zn. Standard Cell Potential For Zinc And Copper.
From www.toppr.com
For the Daniel cell involving the cell reaction Zn(s) + Cu^+2(aq) Zn^+2 Standard Cell Potential For Zinc And Copper And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. The 2 ionic half equations of the cell are given below. Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e. In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the. Standard Cell Potential For Zinc And Copper.
From www.chegg.com
Solved Calculate the standard cell potential of the Standard Cell Potential For Zinc And Copper The cell potential difference is shown with the polarity of the right hand electrode; Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The 2 ionic. Standard Cell Potential For Zinc And Copper.
From slideplayer.com
ELECTROCHEMISTRY. ppt download Standard Cell Potential For Zinc And Copper The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at. Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195602 Standard Cell Potential For Zinc And Copper The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Since zinc is more reactive than copper, zinc loses electrons more readily to. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The cell convention for. Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID1195562 Standard Cell Potential For Zinc And Copper The cell potential difference is shown with the polarity of the right hand electrode; The cell convention for the zinc and copper cell would be; The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive. Standard Cell Potential For Zinc And Copper.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper Standard Cell Potential For Zinc And Copper In 1780, luigi galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the same. Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. And 𝐸 anode is the standard reduction potential of zinc, the. Standard Cell Potential For Zinc And Copper.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Standard Cell Potential For Zinc And Copper The 2 ionic half equations of the cell are given below. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that. Standard Cell Potential For Zinc And Copper.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential For Zinc And Copper To find the difference of. 𝐸 cathode is the standard reduction potential of copper, the cathode, with a value of positive 0.337 volts. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The cell convention for the zinc and copper cell would be; Since zinc is more reactive than copper,. Standard Cell Potential For Zinc And Copper.
From www.pveducation.org
Standard Potential PVEducation Standard Cell Potential For Zinc And Copper Since zinc is more reactive than copper, zinc loses electrons more readily to. The cell potential difference is shown with the polarity of the right hand electrode; The cell convention for the zinc and copper cell would be; And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. The potential of the cell under. Standard Cell Potential For Zinc And Copper.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Standard Cell Potential For Zinc And Copper Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. Zn (s)∣zn 2+ (aq) ∥cu 2+ (aq)∣cu (s) e. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed. Standard Cell Potential For Zinc And Copper.
From chemistrynotes.com
Cell Potential Calculations and Line Notation Standard Cell Potential For Zinc And Copper Since the tabulated standard electrode potentials are reduction potentials, the one which is most negative will need to be reversed in sign to get. The cell convention for the zinc and copper cell would be; And 𝐸 anode is the standard reduction potential of zinc, the anode, with a value of. 𝐸 cathode is the standard reduction potential of copper,. Standard Cell Potential For Zinc And Copper.