Explain Standard Enthalpy Of Formation With Example . A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states.
from www.numerade.com
A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states.
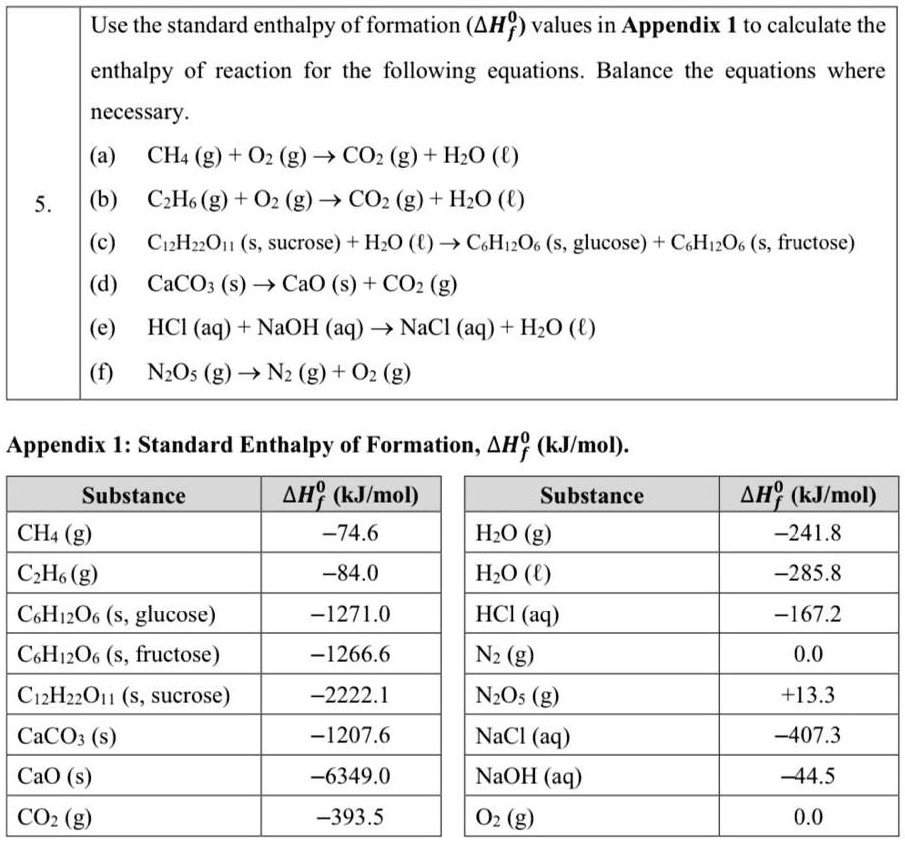
SOLVED Use the standard enthalpy of formation (ΔHf) values in
Explain Standard Enthalpy Of Formation With Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Explain Standard Enthalpy Of Formation With Example A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193 rows in chemistry. Explain Standard Enthalpy Of Formation With Example.
From www.vrogue.co
Hess S Law And Enthalpy Change Calculations vrogue.co Explain Standard Enthalpy Of Formation With Example A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation of any element in its standard. Explain Standard Enthalpy Of Formation With Example.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.2 Standard Enthalpy Change翰林国际教育 Explain Standard Enthalpy Of Formation With Example Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which. Explain Standard Enthalpy Of Formation With Example.
From www.youtube.com
Standard enthalpy of formation example question YouTube Explain Standard Enthalpy Of Formation With Example A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard. Explain Standard Enthalpy Of Formation With Example.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Explain Standard Enthalpy Of Formation With Example The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is defined as the. Explain Standard Enthalpy Of Formation With Example.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Explain Standard Enthalpy Of Formation With Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements. Explain Standard Enthalpy Of Formation With Example.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Explain Standard Enthalpy Of Formation With Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy of formation is defined as the enthalpy change when one. Explain Standard Enthalpy Of Formation With Example.
From www.numerade.com
SOLVED Using the standard enthalpies of formation found in the Explain Standard Enthalpy Of Formation With Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a. Explain Standard Enthalpy Of Formation With Example.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Explain Standard Enthalpy Of Formation With Example The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. The standard enthalpy of formation of any element in its standard state is. Explain Standard Enthalpy Of Formation With Example.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Explain Standard Enthalpy Of Formation With Example The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is defined as the change in enthalpy when one. Explain Standard Enthalpy Of Formation With Example.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Explain Standard Enthalpy Of Formation With Example Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation of any element in its standard state is. Explain Standard Enthalpy Of Formation With Example.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Explain Standard Enthalpy Of Formation With Example The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Explain Standard Enthalpy Of Formation With Example.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Explain Standard Enthalpy Of Formation With Example A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy. Explain Standard Enthalpy Of Formation With Example.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in Explain Standard Enthalpy Of Formation With Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation of any. Explain Standard Enthalpy Of Formation With Example.
From mungfali.com
Standard Enthalpy Change Equation Explain Standard Enthalpy Of Formation With Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is defined as the enthalpy change when one mole of. Explain Standard Enthalpy Of Formation With Example.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Explain Standard Enthalpy Of Formation With Example For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which. Explain Standard Enthalpy Of Formation With Example.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Explain Standard Enthalpy Of Formation With Example Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of any element in its standard state. Explain Standard Enthalpy Of Formation With Example.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Explain Standard Enthalpy Of Formation With Example The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Explain Standard Enthalpy Of Formation With Example.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Explain Standard Enthalpy Of Formation With Example Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics, the standard. Explain Standard Enthalpy Of Formation With Example.
From mavink.com
Enthalpy Of Formation Equation Explain Standard Enthalpy Of Formation With Example Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when. Explain Standard Enthalpy Of Formation With Example.
From chemistry.stackexchange.com
thermodynamics Calculating Enthalpy of formation versus Calculating Explain Standard Enthalpy Of Formation With Example The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Explain Standard Enthalpy Of Formation With Example.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Explain Standard Enthalpy Of Formation With Example A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change. Explain Standard Enthalpy Of Formation With Example.
From people.chem.umass.edu
to Adobe GoLive 6 Explain Standard Enthalpy Of Formation With Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is. Explain Standard Enthalpy Of Formation With Example.
From lessonlistkilderkins.z22.web.core.windows.net
How To Calculate The Enthalpy Explain Standard Enthalpy Of Formation With Example The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy of formation (or heat of formation),. Explain Standard Enthalpy Of Formation With Example.
From studylib.net
Standard Enthalpy of Formation and Reaction Explain Standard Enthalpy Of Formation With Example A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is. Explain Standard Enthalpy Of Formation With Example.
From www.writework.com
A comparison between the Enthalpy of formation of MgO acquired via a Explain Standard Enthalpy Of Formation With Example Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Explain Standard Enthalpy Of Formation With Example.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Explain Standard Enthalpy Of Formation With Example The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from. Explain Standard Enthalpy Of Formation With Example.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Explain Standard Enthalpy Of Formation With Example Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is defined as. Explain Standard Enthalpy Of Formation With Example.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Explain Standard Enthalpy Of Formation With Example Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation \ (δh^\circ_\ce {f}\). Explain Standard Enthalpy Of Formation With Example.
From schoolworkhelper.net
Standard Enthalpies of Formation, ΔH°f SchoolWorkHelper Explain Standard Enthalpy Of Formation With Example The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is defined as. Explain Standard Enthalpy Of Formation With Example.
From www.slideserve.com
PPT Example using enthalpy of combustion and formation PowerPoint Explain Standard Enthalpy Of Formation With Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction. Explain Standard Enthalpy Of Formation With Example.
From www.youtube.com
5.7 Standard Enthalpies of Formation YouTube Explain Standard Enthalpy Of Formation With Example A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of. Explain Standard Enthalpy Of Formation With Example.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Explain Standard Enthalpy Of Formation With Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3), atomic. Explain Standard Enthalpy Of Formation With Example.
From www.numerade.com
A second CFC used as a refrigerant and in aerosols (besides that Explain Standard Enthalpy Of Formation With Example Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. For example, although oxygen can. Explain Standard Enthalpy Of Formation With Example.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Explain Standard Enthalpy Of Formation With Example A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of. Explain Standard Enthalpy Of Formation With Example.