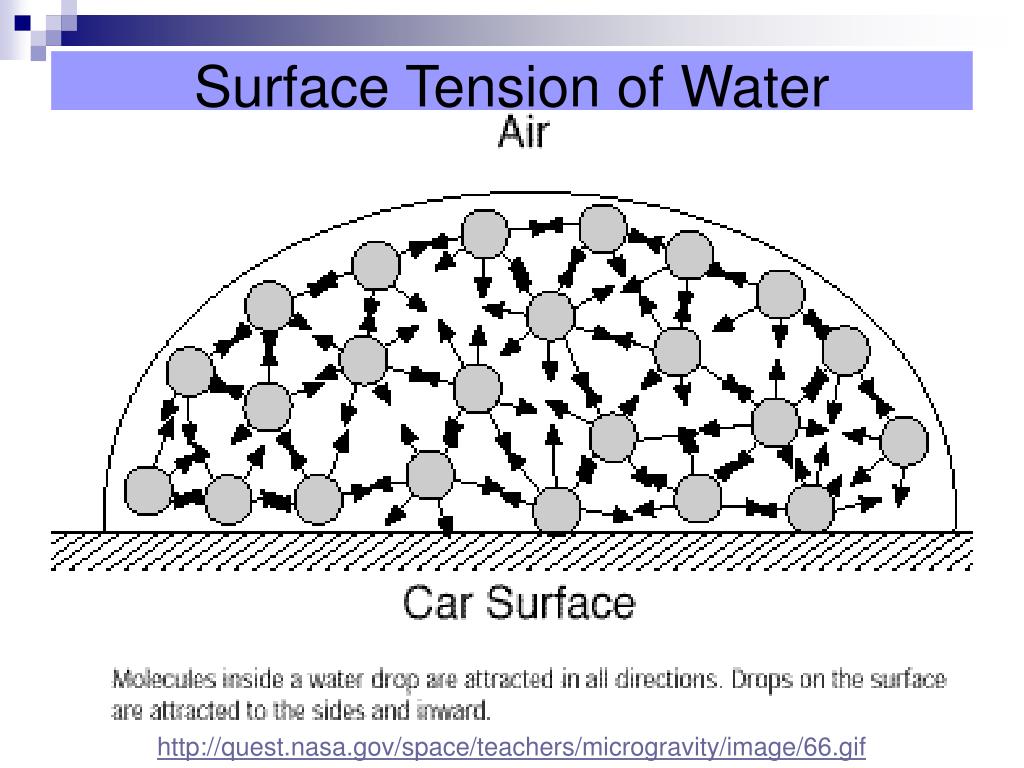
What Is Surface Tension Of A Water Molecule . It would take a force of 72 dynes to break a surface film of water 1 cm long. The molecules at the surface of a glass of water do not have. An example of such an. The attraction of molecules at the surface of a liquid is called surface tension. The surface tension of water decreases significantly with. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The surface tension of water is 72 dynes/cm at 25°c. The polarity of water molecules can help explain why water has a strong surface tension.
from www.slideserve.com
The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. It would take a force of 72 dynes to break a surface film of water 1 cm long. The molecules at the surface of a glass of water do not have. The surface tension of water decreases significantly with. An example of such an. The surface tension of water is 72 dynes/cm at 25°c. The polarity of water molecules can help explain why water has a strong surface tension. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ).
PPT Water and Aqueous Systems PowerPoint Presentation ID565121
What Is Surface Tension Of A Water Molecule The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). The molecules at the surface of a glass of water do not have. The surface tension of water is 72 dynes/cm at 25°c. The polarity of water molecules can help explain why water has a strong surface tension. It would take a force of 72 dynes to break a surface film of water 1 cm long. The attraction of molecules at the surface of a liquid is called surface tension. The surface tension of water decreases significantly with. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. An example of such an.
From ar.inspiredpencil.com
Surface Tension Water What Is Surface Tension Of A Water Molecule The molecules at the surface of a glass of water do not have. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The surface tension of water decreases significantly with. The attraction of molecules at the surface of a liquid is called surface tension. The surface tension of water is 72 dynes/cm at 25°c.. What Is Surface Tension Of A Water Molecule.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector What Is Surface Tension Of A Water Molecule The attraction of molecules at the surface of a liquid is called surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface. What Is Surface Tension Of A Water Molecule.
From errantscience.com
Water surface tension ErrantScience What Is Surface Tension Of A Water Molecule The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The surface tension of water decreases significantly with. The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. The molecules at the surface of a glass of. What Is Surface Tension Of A Water Molecule.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Is Surface Tension Of A Water Molecule The polarity of water molecules can help explain why water has a strong surface tension. The surface tension of water is 72 dynes/cm at 25°c. The molecules at the surface of a glass of water do not have. The attraction of molecules at the surface of a liquid is called surface tension. It would take a force of 72 dynes. What Is Surface Tension Of A Water Molecule.
From www.writework.com
Surface Tension WriteWork What Is Surface Tension Of A Water Molecule The surface tension of water is 72 dynes/cm at 25°c. The molecules at the surface of a glass of water do not have. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The cohesive forces between liquid molecules. What Is Surface Tension Of A Water Molecule.
From www.numerade.com
SOLVED To help explain surface tension, use a single water molecule in What Is Surface Tension Of A Water Molecule The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with. An example of such an. The polarity of water molecules can help explain why water has a strong surface tension. The attraction of molecules at the surface of a liquid is called surface tension. The molecules at the surface of a glass. What Is Surface Tension Of A Water Molecule.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Is Surface Tension Of A Water Molecule The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The surface tension of water is 72 dynes/cm at 25°c. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). The surface tension of water decreases significantly with. It would take. What Is Surface Tension Of A Water Molecule.
From learnwithresikasivakumar.blogspot.com
What is surface tension? What Is Surface Tension Of A Water Molecule The attraction of molecules at the surface of a liquid is called surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension.. What Is Surface Tension Of A Water Molecule.
From errantscience.com
Water surface tension ErrantScience What Is Surface Tension Of A Water Molecule The attraction of molecules at the surface of a liquid is called surface tension. The surface tension of water decreases significantly with. The molecules at the surface of a glass of water do not have. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension of water can cause things. What Is Surface Tension Of A Water Molecule.
From mavink.com
Water Molecules Surface Tension What Is Surface Tension Of A Water Molecule The molecules at the surface of a glass of water do not have. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water. What Is Surface Tension Of A Water Molecule.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 What Is Surface Tension Of A Water Molecule The molecules at the surface of a glass of water do not have. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. An. What Is Surface Tension Of A Water Molecule.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What Is Surface Tension Of A Water Molecule The surface tension of water is 72 dynes/cm at 25°c. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The attraction of molecules at the surface of a liquid is called surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles. What Is Surface Tension Of A Water Molecule.
From worksheetlibleglens.z13.web.core.windows.net
Tension And Surface Tension What Is Surface Tension Of A Water Molecule The polarity of water molecules can help explain why water has a strong surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. It would take a force of 72 dynes to break a surface film of. What Is Surface Tension Of A Water Molecule.
From www.tandfonline.com
Calculation of the surface tension of water 40 years of molecular What Is Surface Tension Of A Water Molecule It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). The polarity of water molecules can help explain why water has a strong surface tension. The surface tension. What Is Surface Tension Of A Water Molecule.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co What Is Surface Tension Of A Water Molecule The polarity of water molecules can help explain why water has a strong surface tension. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with. The molecules at the surface of a glass of. What Is Surface Tension Of A Water Molecule.
From ar.inspiredpencil.com
Surface Tension Water What Is Surface Tension Of A Water Molecule An example of such an. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of. What Is Surface Tension Of A Water Molecule.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts What Is Surface Tension Of A Water Molecule Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). The polarity of water molecules can help explain why water has a strong surface tension. The molecules at the surface of a glass of water do not have. “surface tension is the tension of a liquid’s. What Is Surface Tension Of A Water Molecule.
From www.researchgate.net
2 Schematic representation of surface tension of a hemispherical water What Is Surface Tension Of A Water Molecule The surface tension of water is 72 dynes/cm at 25°c. The molecules at the surface of a glass of water do not have. The polarity of water molecules can help explain why water has a strong surface tension. It would take a force of 72 dynes to break a surface film of water 1 cm long. The attraction of molecules. What Is Surface Tension Of A Water Molecule.
From www.usgs.gov
The strong polar bond between water molecules creates water cohesion. What Is Surface Tension Of A Water Molecule The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The polarity of water molecules can help explain why water has a strong surface tension. The attraction. What Is Surface Tension Of A Water Molecule.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Is Surface Tension Of A Water Molecule The surface tension of water is 72 dynes/cm at 25°c. The attraction of molecules at the surface of a liquid is called surface tension. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water decreases significantly with. Surface tension of water can cause things to float which. What Is Surface Tension Of A Water Molecule.
From www.expii.com
Water — Molecular Structure & Bonding Expii What Is Surface Tension Of A Water Molecule The molecules at the surface of a glass of water do not have. The surface tension of water decreases significantly with. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The attraction of molecules at the surface of a liquid is called surface tension. An example of such an. The surface tension of water. What Is Surface Tension Of A Water Molecule.
From ar.inspiredpencil.com
Surface Tension Water What Is Surface Tension Of A Water Molecule “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water decreases significantly with. The attraction of. What Is Surface Tension Of A Water Molecule.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Is Surface Tension Of A Water Molecule An example of such an. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water is 72 dynes/cm at 25°c. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface of a glass of water do not. What Is Surface Tension Of A Water Molecule.
From www.acs.org
Simulations & Videos for Lesson 5.2 Surface Tension American What Is Surface Tension Of A Water Molecule An example of such an. The attraction of molecules at the surface of a liquid is called surface tension. It would take a force of 72 dynes to break a surface film of water 1 cm long. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the. What Is Surface Tension Of A Water Molecule.
From www.researchgate.net
Surface tension and capillary forces acting on papermaking fibres What Is Surface Tension Of A Water Molecule The surface tension of water decreases significantly with. An example of such an. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The polarity of water molecules can help explain why water has a strong surface tension. The. What Is Surface Tension Of A Water Molecule.
From www.youtube.com
Surface Tension of Water Explained YouTube What Is Surface Tension Of A Water Molecule The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. The polarity of water molecules can help explain why water has a strong surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s. What Is Surface Tension Of A Water Molecule.
From www.myxxgirl.com
Ppt Lesson Cohesion Adhesion Surface Tension Powerpoint My XXX Hot Girl What Is Surface Tension Of A Water Molecule It would take a force of 72 dynes to break a surface film of water 1 cm long. The polarity of water molecules can help explain why water has a strong surface tension. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension of water can cause things to float which are denser. What Is Surface Tension Of A Water Molecule.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts What Is Surface Tension Of A Water Molecule The attraction of molecules at the surface of a liquid is called surface tension. The surface tension of water is 72 dynes/cm at 25°c. An example of such an. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms. What Is Surface Tension Of A Water Molecule.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Is Surface Tension Of A Water Molecule It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water decreases significantly with. The polarity of water molecules can help explain why water has a strong surface tension. The attraction of molecules at the surface of a liquid is called surface tension. The cohesive forces between liquid. What Is Surface Tension Of A Water Molecule.
From errantscience.com
Water surface tension ErrantScience What Is Surface Tension Of A Water Molecule It would take a force of 72 dynes to break a surface film of water 1 cm long. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules at the surface of a glass of water do. What Is Surface Tension Of A Water Molecule.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces What Is Surface Tension Of A Water Molecule The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The polarity of water molecules can help explain why water has a strong surface tension. The surface tension of water decreases significantly with. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. What Is Surface Tension Of A Water Molecule.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules What Is Surface Tension Of A Water Molecule An example of such an. The surface tension of water is 72 dynes/cm at 25°c. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). The polarity of water molecules can help explain why water has a strong surface tension. The molecules at the surface of. What Is Surface Tension Of A Water Molecule.
From www.pinterest.com
Surface tension, Surface, Tension What Is Surface Tension Of A Water Molecule The attraction of molecules at the surface of a liquid is called surface tension. An example of such an. The surface tension of water decreases significantly with. The polarity of water molecules can help explain why water has a strong surface tension. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. It would take. What Is Surface Tension Of A Water Molecule.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Is Surface Tension Of A Water Molecule It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). An example of such an. The molecules at the surface of a glass of water do not have.. What Is Surface Tension Of A Water Molecule.
From commons.wikimedia.org
FileSurface Tension 01.jpg What Is Surface Tension Of A Water Molecule The surface tension of water decreases significantly with. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. An example of such an. The molecules at the surface of a glass of water do not have. The attraction of molecules at the surface of a liquid is called surface tension. The polarity of water molecules. What Is Surface Tension Of A Water Molecule.