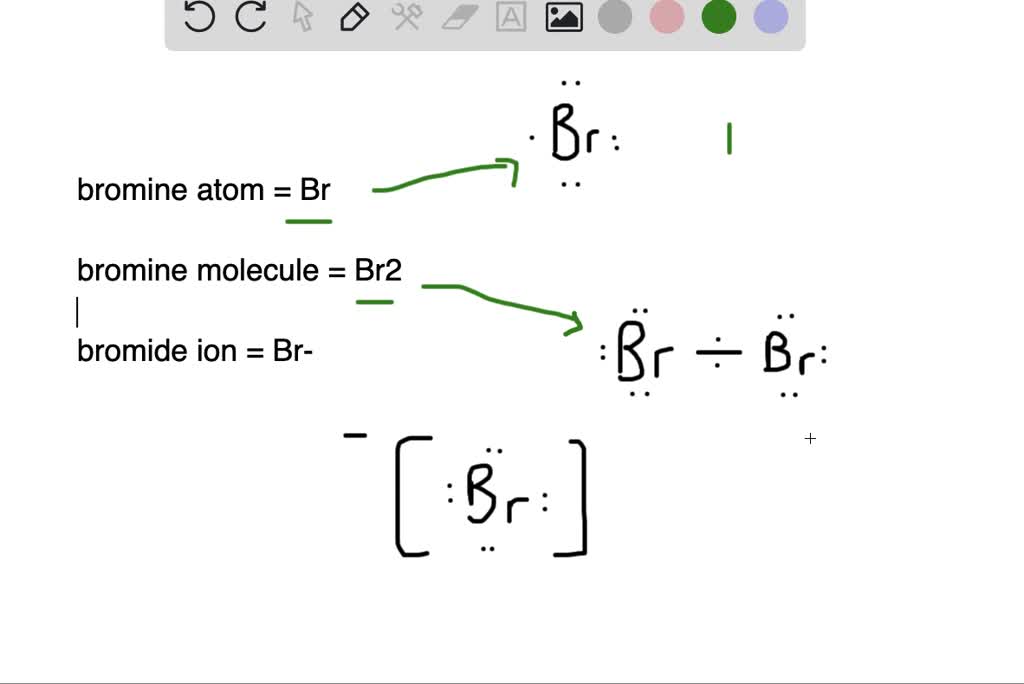
Bromine Unpaired Electrons . In the periodic table, bromine is a group viia element with seven electrons in. One lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Allotropes some elements exist in several different structural forms, called allotropes. One lone pair and three unpaired electrons. when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. the bromine molecule contains only one element. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol:
from www.numerade.com
group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: One lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. One lone pair and three unpaired electrons. Allotropes some elements exist in several different structural forms, called allotropes. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. the bromine molecule contains only one element. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical.
What is the difference between (a) a bromine atom, (b) a bromine
Bromine Unpaired Electrons Allotropes some elements exist in several different structural forms, called allotropes. One lone pair and three unpaired electrons. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). One lone pair and three unpaired electrons. In the periodic table, bromine is a group viia element with seven electrons in. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. the bromine molecule contains only one element. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Allotropes some elements exist in several different structural forms, called allotropes.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Bromine Unpaired Electrons One lone pair and three unpaired electrons. the bromine molecule contains only one element. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: One lone pair and three unpaired electrons. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. when the carbon. Bromine Unpaired Electrons.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Unpaired Electrons the bromine molecule contains only one element. when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. In the periodic table, bromine is a group viia element with seven electrons in. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7.. Bromine Unpaired Electrons.
From techschematic.com
Understanding the Electron Dot Diagram for Bromine A Visual Bromine Unpaired Electrons the bromine molecule contains only one element. Allotropes some elements exist in several different structural forms, called allotropes. when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: The simplest example of alkyl radical. Bromine Unpaired Electrons.
From cewsmrrv.blob.core.windows.net
Bromine Has Valence Electrons And Inner (Core) Electrons at Dora Dupre blog Bromine Unpaired Electrons The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. One lone pair and three unpaired electrons. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. One lone pair and three unpaired electrons. when the carbon atom of a alkyl group. Bromine Unpaired Electrons.
From app.emaze.com
Bromine ) on emaze Bromine Unpaired Electrons One lone pair and three unpaired electrons. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia).. Bromine Unpaired Electrons.
From www.dreamstime.com
Element of Bromine stock vector. Illustration of college 104400483 Bromine Unpaired Electrons One lone pair and three unpaired electrons. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: the bromine molecule contains only one element. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. group 15 elements such as nitrogen have five valence electrons. Bromine Unpaired Electrons.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and Bromine Unpaired Electrons In the periodic table, bromine is a group viia element with seven electrons in. One lone pair and three unpaired electrons. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Allotropes some elements exist in several different structural forms, called allotropes. group 15 elements such as nitrogen have five valence electrons in. Bromine Unpaired Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Unpaired Electrons One lone pair and three unpaired electrons. One lone pair and three unpaired electrons. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. Allotropes some elements exist in several different structural forms, called allotropes. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). . Bromine Unpaired Electrons.
From diagramdataconfusion.z22.web.core.windows.net
Electron Dot Diagram For Bromine Bromine Unpaired Electrons The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). One lone pair and three unpaired electrons. group 15. Bromine Unpaired Electrons.
From mavink.com
Free Radical Lewis Structure Bromine Unpaired Electrons Allotropes some elements exist in several different structural forms, called allotropes. In the periodic table, bromine is a group viia element with seven electrons in. One lone pair and three unpaired electrons. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. group 15 elements such as nitrogen have five. Bromine Unpaired Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Unpaired Electrons group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: One lone pair and three unpaired electrons. One lone pair and three unpaired electrons. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus. Bromine Unpaired Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Unpaired Electrons In the periodic table, bromine is a group viia element with seven electrons in. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: One lone pair and three unpaired electrons. when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. The simplest example of. Bromine Unpaired Electrons.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library Bromine Unpaired Electrons based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Allotropes some elements exist in several different structural forms, called allotropes. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). In the periodic table, bromine is a group viia element with seven electrons in. when. Bromine Unpaired Electrons.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Unpaired Electrons The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: group 15 elements such as. Bromine Unpaired Electrons.
From schematicdatavenin77.z5.web.core.windows.net
Lewis Electron Dot Symbol For Bromine Bromine Unpaired Electrons group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: One lone pair and three unpaired electrons. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. the bromine molecule contains only one element. when the carbon atom of a alkyl group has. Bromine Unpaired Electrons.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine Unpaired Electrons One lone pair and three unpaired electrons. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Allotropes some elements exist in several different structural forms, called allotropes. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). the bromine molecule contains only one element. In. Bromine Unpaired Electrons.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine Unpaired Electrons One lone pair and three unpaired electrons. Allotropes some elements exist in several different structural forms, called allotropes. the bromine molecule contains only one element. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has. Bromine Unpaired Electrons.
From www.alamy.com
Bromine molecular model Stock Vector Images Alamy Bromine Unpaired Electrons the bromine molecule contains only one element. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl. Bromine Unpaired Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Unpaired Electrons Allotropes some elements exist in several different structural forms, called allotropes. In the periodic table, bromine is a group viia element with seven electrons in. the bromine molecule contains only one element. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: To obtain an octet, these atoms form three covalent bonds, as. Bromine Unpaired Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Unpaired Electrons To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Allotropes some elements exist in several different structural forms, called allotropes. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. the bromine molecule contains only one element. group 15 elements such as nitrogen have. Bromine Unpaired Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Unpaired Electrons The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: the bromine molecule contains only one element. One lone pair and three unpaired electrons. In the periodic table, bromine is a group viia element. Bromine Unpaired Electrons.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Unpaired Electrons based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. the bromine molecule contains only one element. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: In the periodic table, bromine is a group viia element with seven electrons in. To obtain an octet,. Bromine Unpaired Electrons.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Unpaired Electrons the bromine molecule contains only one element. Allotropes some elements exist in several different structural forms, called allotropes. One lone pair and three unpaired electrons. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: One lone pair and three unpaired electrons. group 15 elements such as nitrogen have five valence electrons. Bromine Unpaired Electrons.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Unpaired Electrons One lone pair and three unpaired electrons. when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: the bromine molecule contains only one element. One lone pair and three unpaired electrons. group 15. Bromine Unpaired Electrons.
From www.slideserve.com
PPT Topic Chemistry Aim Explain how elements are classified in Bromine Unpaired Electrons In the periodic table, bromine is a group viia element with seven electrons in. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). the bromine molecule contains only one element. when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. The simplest example of. Bromine Unpaired Electrons.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Unpaired Electrons In the periodic table, bromine is a group viia element with seven electrons in. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. Allotropes some elements exist in several different structural forms, called allotropes. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3. Bromine Unpaired Electrons.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Bromine Unpaired Electrons the bromine molecule contains only one element. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: when the carbon atom of a alkyl group has an unpaired electron, the species is the. Bromine Unpaired Electrons.
From www.shutterstock.com
Bohr Model Bromine Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่า Bromine Unpaired Electrons group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). In the periodic table, bromine is a group viia element with seven electrons. Bromine Unpaired Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Unpaired Electrons To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). One lone pair and three unpaired electrons. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: the bromine molecule contains only one element. group 15 elements such as nitrogen have five valence electrons in the atomic. Bromine Unpaired Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Unpaired Electrons One lone pair and three unpaired electrons. Allotropes some elements exist in several different structural forms, called allotropes. One lone pair and three unpaired electrons. the bromine molecule contains only one element. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. To obtain an octet, these atoms form three. Bromine Unpaired Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Unpaired Electrons One lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). the bromine molecule contains only one element. Allotropes some elements exist in several different structural forms, called allotropes. One lone pair and three unpaired electrons. In the periodic table, bromine is a group viia element with seven. Bromine Unpaired Electrons.
From www.istockphoto.com
Br Bromine Element Information Facts Properties Trends Uses And Bromine Unpaired Electrons when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). The simplest example of alkyl radical is •ch. Bromine Unpaired Electrons.
From www.numerade.com
SOLVED For the Bromine atom a) Determine the total number of unpaired Bromine Unpaired Electrons group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: In the periodic table, bromine is a group viia element with seven electrons in. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. One lone pair and three unpaired electrons. Allotropes some elements exist in. Bromine Unpaired Electrons.
From dxooqbere.blob.core.windows.net
Bromine Configuration Of Electrons at Daniel Wright blog Bromine Unpaired Electrons group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: the bromine molecule contains only one element. The simplest example of alkyl radical is •ch 3 , with the total number of valence electron as 7. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: One. Bromine Unpaired Electrons.
From www.numerade.com
SOLVEDWrite orbital diagrams for the valence electrons and indicate Bromine Unpaired Electrons In the periodic table, bromine is a group viia element with seven electrons in. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: when the carbon atom of a alkyl group has an unpaired electron, the species is the alkyl radical. Allotropes some elements exist in several different structural forms, called allotropes.. Bromine Unpaired Electrons.