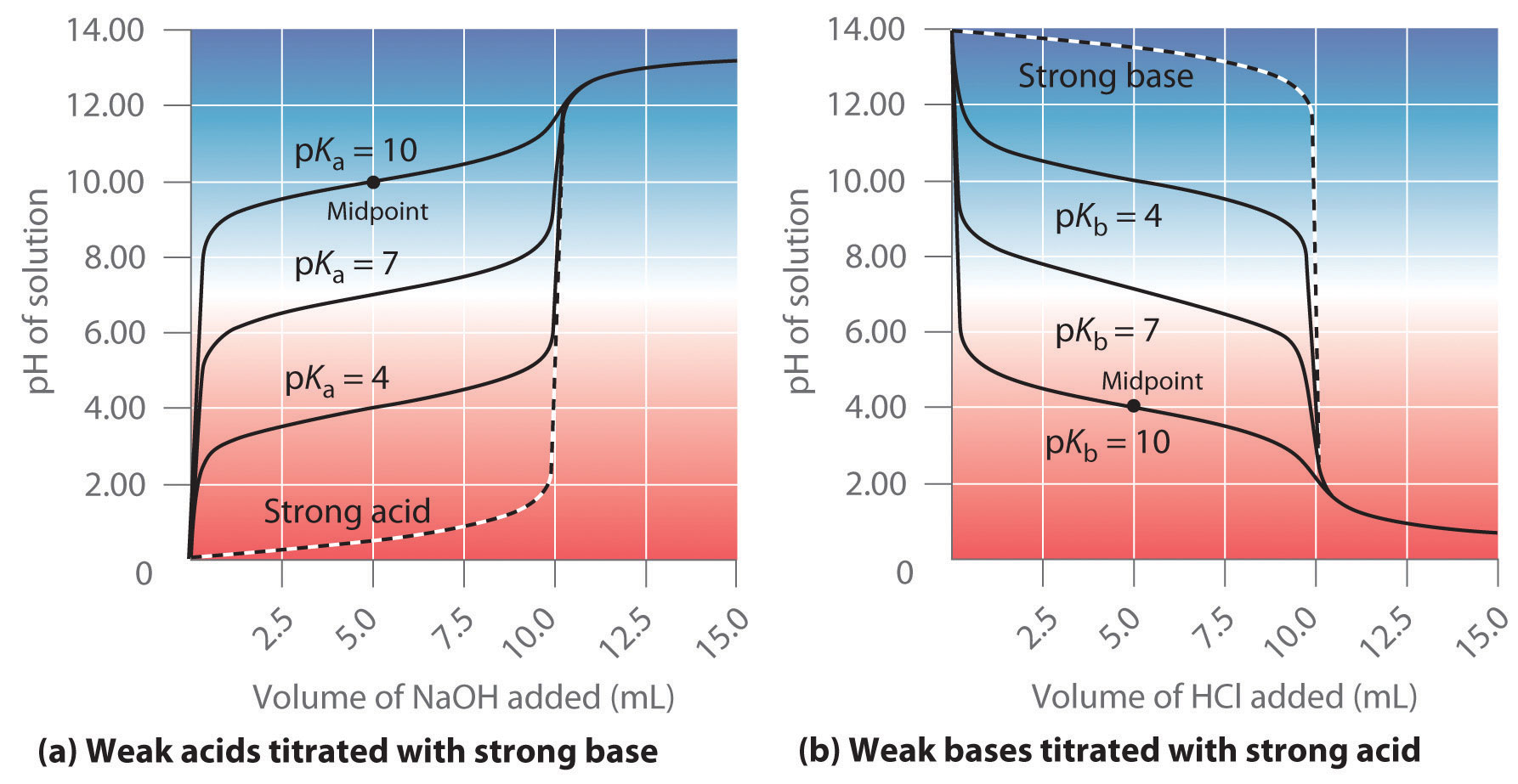
Titration Curve Maximum Buffering Capacity . Understand the relationship between the. This is known as the buffer zone. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. Example of weak acid titration curve. When a small amount of acid or base is added to the buffer system, the curve will increase dramatically once it exits the buffer region. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. However, addition of too big amount of an acid. at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. a titration curve graphically depicts buffer capacity. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by.
from exoyzonai.blob.core.windows.net
a titration curve graphically depicts buffer capacity. However, addition of too big amount of an acid. This is known as the buffer zone. at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. Example of weak acid titration curve. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. When a small amount of acid or base is added to the buffer system, the curve will increase dramatically once it exits the buffer region. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. Understand the relationship between the. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by.
Titration Curve Labeled Buffer Region at Craig Johnson blog
Titration Curve Maximum Buffering Capacity Example of weak acid titration curve. This is known as the buffer zone. a titration curve graphically depicts buffer capacity. Understand the relationship between the. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. Example of weak acid titration curve. However, addition of too big amount of an acid. at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. When a small amount of acid or base is added to the buffer system, the curve will increase dramatically once it exits the buffer region.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. a titration curve graphically depicts buffer capacity. Example of weak acid titration curve. Titration of acetic acid solution (pk a = 4.7). Titration Curve Maximum Buffering Capacity.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titration Curve Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer. Titration Curve Maximum Buffering Capacity.
From mavink.com
Buffer Region Titration Curve Titration Curve Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. This is known as the buffer zone. a titration curve graphically depicts buffer capacity. The middle of the curve is flat because the addition of base or acid has. Titration Curve Maximum Buffering Capacity.
From www.coursehero.com
[Solved] Where is my buffering regions? Why do the titration curves of Titration Curve Maximum Buffering Capacity at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. However, addition of too big amount of an acid. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by.. Titration Curve Maximum Buffering Capacity.
From chemistryguru.com.sg
Titration Curve for Weak Acid Strong Base Titration Curve Maximum Buffering Capacity When a small amount of acid or base is added to the buffer system, the curve will increase dramatically once it exits the buffer region. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. in more rigorous terms, buffer capacity is defined as the number. Titration Curve Maximum Buffering Capacity.
From www.slideshare.net
02 hydrolysis. buffers__colloids Titration Curve Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. However, addition of too big amount of. Titration Curve Maximum Buffering Capacity.
From www.researchgate.net
Acid or base titration curves and buffering capacity of reference Titration Curve Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. However, addition of too big amount of an acid. This is known as the buffer zone. When a small amount of acid or. Titration Curve Maximum Buffering Capacity.
From ceckgokq.blob.core.windows.net
Titration Curve In Biochemistry at William Speece blog Titration Curve Maximum Buffering Capacity a titration curve graphically depicts buffer capacity. However, addition of too big amount of an acid. Understand the relationship between the. When a small amount of acid or base is added to the buffer system, the curve will increase dramatically once it exits the buffer region. Example of weak acid titration curve. The middle of the curve is flat. Titration Curve Maximum Buffering Capacity.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Weak Acids and Buffers Titration Curve Maximum Buffering Capacity a titration curve graphically depicts buffer capacity. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. This is known as the buffer zone. When a small amount of acid or base is added to the buffer system, the. Titration Curve Maximum Buffering Capacity.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Curve Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. However, addition of too big amount of an acid. When a small amount of acid or base is added to the buffer system,. Titration Curve Maximum Buffering Capacity.
From www.youtube.com
Buffers and Titration Curves YouTube Titration Curve Maximum Buffering Capacity a titration curve graphically depicts buffer capacity. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. However, addition of too big amount of an acid. The middle of the curve is flat because the addition of base or. Titration Curve Maximum Buffering Capacity.
From riaquueta45.blogspot.com
How To Determine Buffer Capacity From A Titration Curve Gerald Hipple Titration Curve Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. This is known as the buffer zone. The middle of the curve is flat because the addition of base or acid has little. Titration Curve Maximum Buffering Capacity.
From derangedphysiology.com
Buffers and buffering power Deranged Physiology Titration Curve Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. a titration curve graphically depicts buffer capacity. The middle. Titration Curve Maximum Buffering Capacity.
From byjus.com
What is the significance of maximum buffer capacity in the case of weak Titration Curve Maximum Buffering Capacity Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. When a small amount of acid or base is added to the buffer system, the curve will increase dramatically once it exits the buffer region. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the. Titration Curve Maximum Buffering Capacity.
From www.researchgate.net
Acid or base titration curves and buffering capacity of reference Titration Curve Maximum Buffering Capacity This is known as the buffer zone. Understand the relationship between the. When a small amount of acid or base is added to the buffer system, the curve will increase dramatically once it exits the buffer region. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be. Titration Curve Maximum Buffering Capacity.
From www.researchgate.net
Acid or base titration curves and buffering capacity of polypeptide Titration Curve Maximum Buffering Capacity However, addition of too big amount of an acid. a titration curve graphically depicts buffer capacity. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. Understand the relationship between the. Example. Titration Curve Maximum Buffering Capacity.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts Titration Curve Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. a titration curve graphically depicts buffer capacity. the buffer capacity is the amount of acid or base that can be added. Titration Curve Maximum Buffering Capacity.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Curve Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution.. Titration Curve Maximum Buffering Capacity.
From mungfali.com
Titration Curve Graph Titration Curve Maximum Buffering Capacity This is known as the buffer zone. Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. in more rigorous terms, buffer capacity is defined as. Titration Curve Maximum Buffering Capacity.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Curve Maximum Buffering Capacity at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. However, addition. Titration Curve Maximum Buffering Capacity.
From pilgaard.info
Acids and bases Buffers Michael Pilgaard's Chemistry Titration Curve Maximum Buffering Capacity a titration curve graphically depicts buffer capacity. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually. Titration Curve Maximum Buffering Capacity.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Maximum Buffering Capacity Example of weak acid titration curve. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. a titration curve graphically depicts buffer capacity. However, addition of too big amount of an acid. in more rigorous terms, buffer capacity is defined as the number of moles. Titration Curve Maximum Buffering Capacity.
From www.chegg.com
PART DETERMINING THE EFFECT O C. TITRATION OF BUFFERS Titration Curve Maximum Buffering Capacity at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. This is known as the buffer zone. Example of weak acid titration curve. However, addition of too big amount of an acid. a titration curve graphically depicts buffer capacity. Understand the relationship between the. in more rigorous. Titration Curve Maximum Buffering Capacity.
From www.researchgate.net
Buffer capacity curve for a given mixture of TNH, TNO2, TIC and TIP in Titration Curve Maximum Buffering Capacity Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. a titration curve graphically depicts buffer capacity. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or. Titration Curve Maximum Buffering Capacity.
From www.numerade.com
SOLVED Max Buffering Capacity Unanswered 3 attempts left Mark the Titration Curve Maximum Buffering Capacity Understand the relationship between the. However, addition of too big amount of an acid. Example of weak acid titration curve. Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter. Titration Curve Maximum Buffering Capacity.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Titration Curve Maximum Buffering Capacity When a small amount of acid or base is added to the buffer system, the curve will increase dramatically once it exits the buffer region. Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. However, addition of too big amount of an acid. at this point the buffer solution has its maximum buffer capacity, i.e.,. Titration Curve Maximum Buffering Capacity.
From exosxgjvz.blob.core.windows.net
Weak Acid Titration Curve Buffer Region at Paula Rivera blog Titration Curve Maximum Buffering Capacity However, addition of too big amount of an acid. at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. in more rigorous terms, buffer capacity is defined as the number of moles of an acid. Titration Curve Maximum Buffering Capacity.
From mavink.com
Buffer Region Titration Curve Titration Curve Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. However, addition of too big amount of an acid. Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. at this point the buffer solution has. Titration Curve Maximum Buffering Capacity.
From riaquueta45.blogspot.com
How To Determine Buffer Capacity From A Titration Curve Gerald Hipple Titration Curve Maximum Buffering Capacity Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. However, addition of too big amount of an acid. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. When a small amount of acid or base is added to the buffer system, the. Titration Curve Maximum Buffering Capacity.
From godwinaces1963.blogspot.com
Weak Diprotic Acid Titration Curve Godwin Aces1963 Titration Curve Maximum Buffering Capacity Titration of acetic acid solution (pk a = 4.7) with strong base (e.g. However, addition of too big amount of an acid. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. in more rigorous terms, buffer capacity is defined as the number of moles of. Titration Curve Maximum Buffering Capacity.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titration Curve Maximum Buffering Capacity The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. Example of weak acid titration curve. at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. the buffer capacity is the amount of acid or. Titration Curve Maximum Buffering Capacity.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titration Curve Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. However, addition of too big amount of an acid.. Titration Curve Maximum Buffering Capacity.
From chart-studio.plotly.com
Titration of Phosphate Buffer with Base scatter chart made by Titration Curve Maximum Buffering Capacity When a small amount of acid or base is added to the buffer system, the curve will increase dramatically once it exits the buffer region. This is known as the buffer zone. The middle of the curve is flat because the addition of base or acid has little effect on the ph of the solution. the buffer capacity is. Titration Curve Maximum Buffering Capacity.
From www.researchgate.net
Acid or base titration curves and buffering capacity of target and Titration Curve Maximum Buffering Capacity Understand the relationship between the. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by. a titration curve graphically depicts buffer capacity. However, addition of too big amount of an acid. This is known as the buffer zone. Titration. Titration Curve Maximum Buffering Capacity.
From www.numerade.com
SOLVED Max Buffering Capacity Unanswered 3 attempts left Mark the Titration Curve Maximum Buffering Capacity at this point the buffer solution has its maximum buffer capacity, i.e., the slope of the titration curve has a. Example of weak acid titration curve. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its. Titration Curve Maximum Buffering Capacity.