Two Element X And Y Have Atomic Number 12 And 16 Respectively . • electronic configuration of y : The element x has atomic number 12 and element y has atomic number 16. Magnesium being the metal will tend to lose an. The electronic configuration of x will be 2, 8, 2 and that of y will. Electronic configuration of element 'y' atomic number. Electronic configuration of element x, atomic number 12=2;8;2. What type of bond will be formed. • electronic configuration of x : To which period of the modern periodic table do these two elements belong? Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. To which period of the modern periodic table do these two elements belong? Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. Two elements x and y have atomic numbers 12 and 16 respectively. What type of bond will be formed between them and.
from www.doubtnut.com
• electronic configuration of x : Electronic configuration of element 'y' atomic number. Electronic configuration of element x, atomic number 12=2;8;2. To which period of the modern periodic table do these two elements belong? The electronic configuration of x will be 2, 8, 2 and that of y will. Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. To which period of the modern periodic table do these two elements belong? Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. Magnesium being the metal will tend to lose an. The element x has atomic number 12 and element y has atomic number 16.
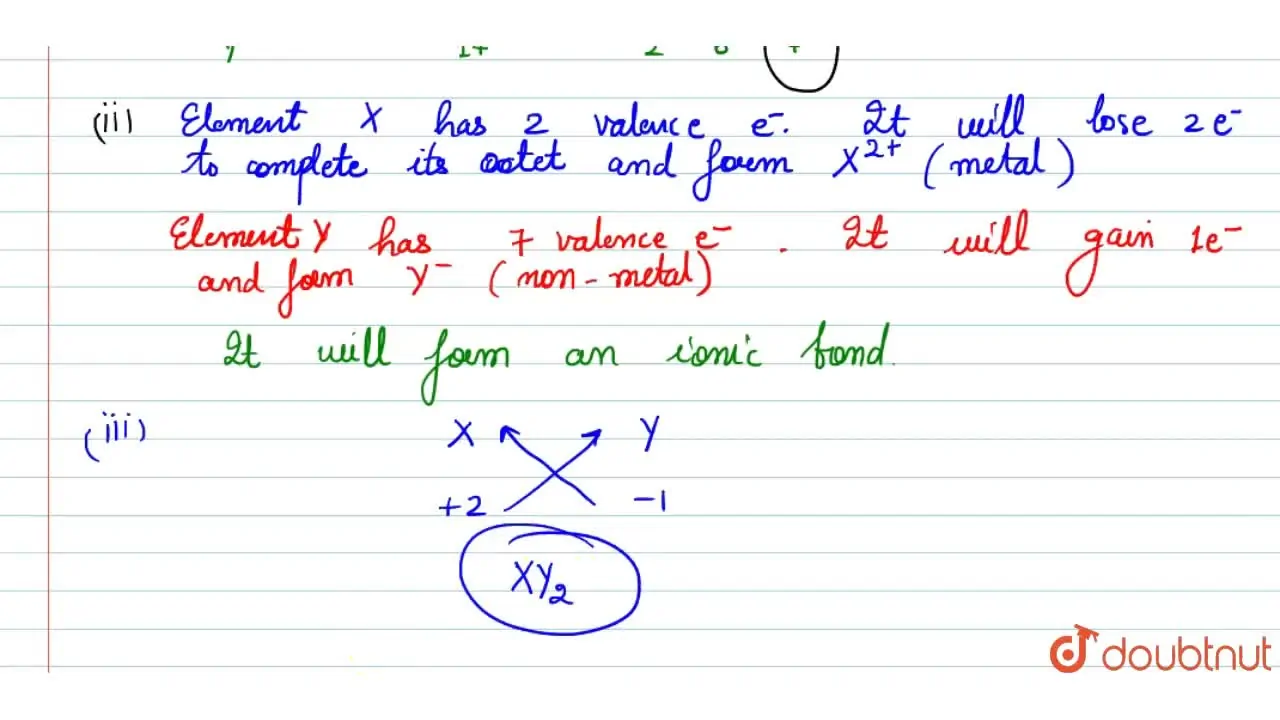
Two elements X and Y have atomic number 12 and 17 respectively. (i)
Two Element X And Y Have Atomic Number 12 And 16 Respectively Magnesium being the metal will tend to lose an. To which period of the modern periodic table do these two elements belong? What type of bond will be formed. Magnesium being the metal will tend to lose an. Electronic configuration of element 'y' atomic number. Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. • electronic configuration of y : The electronic configuration of x will be 2, 8, 2 and that of y will. The element x has atomic number 12 and element y has atomic number 16. Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. Two elements x and y have atomic numbers 12 and 16 respectively. • electronic configuration of x : To which period of the modern periodic table do these two elements belong? Electronic configuration of element x, atomic number 12=2;8;2. What type of bond will be formed between them and.
From www.britannica.com
periodic table Definition, Elements, Groups, Charges, Trends, & Facts Two Element X And Y Have Atomic Number 12 And 16 Respectively To which period of the modern periodic table do these two elements belong? Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. Magnesium being the metal will tend to lose an. Electronic configuration of element x, atomic number 12=2;8;2. To which period of the modern periodic table do these two elements belong? • electronic configuration of y. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From chemistry.about.com
Element List Atomic Number, Element Name and Symbol Two Element X And Y Have Atomic Number 12 And 16 Respectively Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. • electronic configuration of x : To which period of the modern periodic table do these two elements belong? The element x has atomic number 12 and element y has atomic number 16. • electronic configuration of y : What type of bond will be formed. To which. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From utedzz.blogspot.com
Periodic Table Atomic Number 12 Periodic Table Timeline Two Element X And Y Have Atomic Number 12 And 16 Respectively Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. What type of bond will be formed. • electronic configuration of x : • electronic configuration of y : Magnesium being the metal will tend to lose an. Two elements x and y have atomic numbers 12 and. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.thoughtco.com
Element List Atomic Number, Element Name and Symbol Two Element X And Y Have Atomic Number 12 And 16 Respectively What type of bond will be formed. To which period of the modern periodic table do these two elements belong? • electronic configuration of x : Two elements x and y have atomic numbers 12 and 16 respectively. Magnesium being the metal will tend to lose an. What type of bond will be formed between them and. Electronic configuration of. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol Two Element X And Y Have Atomic Number 12 And 16 Respectively What type of bond will be formed. To which period of the modern periodic table do these two elements belong? Magnesium being the metal will tend to lose an. What type of bond will be formed between them and. Electronic configuration of element 'y' atomic number. • electronic configuration of y : Two elements x and y have atomic numbers. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.toppr.com
7. Two elements X and Y have atomic number 11 and 16 respectively. (a Two Element X And Y Have Atomic Number 12 And 16 Respectively The element x has atomic number 12 and element y has atomic number 16. Two elements x and y have atomic numbers 12 and 16 respectively. Electronic configuration of element 'y' atomic number. Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. The electronic configuration of x will be 2, 8, 2. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.doubtnut.com
Two elements X and Y have atomic number 12 and 17 respectively. (i) Two Element X And Y Have Atomic Number 12 And 16 Respectively Electronic configuration of element 'y' atomic number. To which period of the modern periodic table do these two elements belong? The element x has atomic number 12 and element y has atomic number 16. The electronic configuration of x will be 2, 8, 2 and that of y will. Two elements x and y have atomic numbers 12 and 16. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Two Element X And Y Have Atomic Number 12 And 16 Respectively To which period of the modern periodic table do these two elements belong? Electronic configuration of element 'y' atomic number. • electronic configuration of y : What type of bond will be formed between them and. • electronic configuration of x : Electronic configuration of element x, atomic number 12=2;8;2. Two elements $$x$$ and $$y$$ have atomic number $$12$$ and. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.toppr.com
17 Two elements X and Y have atomic numbers 12 and 16 respectively. To Two Element X And Y Have Atomic Number 12 And 16 Respectively Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. What type of bond will be formed. The element x has atomic number 12 and element y has atomic number 16. • electronic configuration of x. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.toppr.com
Elements X, Y, and Z have atomic numbers 6, 9, and 12 respectively Two Element X And Y Have Atomic Number 12 And 16 Respectively Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. To which period of the modern periodic table do these two elements belong? To which period of the modern periodic table. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.doubtnut.com
Two elements X and Y have atomic numbers 12 and 16 respectively. To wh Two Element X And Y Have Atomic Number 12 And 16 Respectively The electronic configuration of x will be 2, 8, 2 and that of y will. The element x has atomic number 12 and element y has atomic number 16. To which period of the modern periodic table do these two elements belong? Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. Both. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.vectorstock.com
Periodic table of the elements with atomic number Vector Image Two Element X And Y Have Atomic Number 12 And 16 Respectively The electronic configuration of x will be 2, 8, 2 and that of y will. What type of bond will be formed between them and. Magnesium being the metal will tend to lose an. To which period of the modern periodic table do these two elements belong? What type of bond will be formed. Two elements $$x$$ and $$y$$ have. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.doubtnut.com
Two elements X and Y have atomic numbers 19 and 17 respectively. Give Two Element X And Y Have Atomic Number 12 And 16 Respectively Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. What type of bond will be formed between them and. To which period of the modern periodic table do these two elements belong? The element x has atomic number 12 and element y has atomic number 16. Two elements $$x$$ and $$y$$ have. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.oceanproperty.co.th
Periodic Table Of Elements With Names And Symbols And Atomic Mass And Two Element X And Y Have Atomic Number 12 And 16 Respectively What type of bond will be formed between them and. The element x has atomic number 12 and element y has atomic number 16. Two elements x and y have atomic numbers 12 and 16 respectively. Electronic configuration of element x, atomic number 12=2;8;2. Electronic configuration of element 'y' atomic number. • electronic configuration of y : To which period. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From mungfali.com
Periodic Table With Names And Atomic Mass Two Element X And Y Have Atomic Number 12 And 16 Respectively To which period of the modern periodic table do these two elements belong? Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. The element x has atomic number 12 and element y has atomic number 16. Two elements x and y have atomic numbers 12 and 16 respectively. To which period of the modern periodic table do. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From utedzz.blogspot.com
Periodic Table Atomic Number 12 Periodic Table Timeline Two Element X And Y Have Atomic Number 12 And 16 Respectively Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. To which period of the modern periodic table do these two elements belong? Magnesium being the metal will tend to lose an. Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From brainly.in
an element x has atomic number 12 and an atomic mass number 26 . draw a Two Element X And Y Have Atomic Number 12 And 16 Respectively Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. The element x has atomic number 12 and element y has atomic number 16. What type of bond will be formed between them and. Two elements x and y have atomic numbers 12 and 16 respectively. The electronic configuration of x will be. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From sciencenotes.org
Periodic Table of Element Atom Sizes Two Element X And Y Have Atomic Number 12 And 16 Respectively Two elements x and y have atomic numbers 12 and 16 respectively. Electronic configuration of element 'y' atomic number. • electronic configuration of y : Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. To which period of the modern periodic table do these two elements belong?. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.toppr.com
The atomic numbers of two elements A and B are 11 and 12 respectively Two Element X And Y Have Atomic Number 12 And 16 Respectively The element x has atomic number 12 and element y has atomic number 16. • electronic configuration of x : To which period of the modern periodic table do these two elements belong? Magnesium being the metal will tend to lose an. To which period of the modern periodic table do these two elements belong? Electronic configuration of element 'y'. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.toppr.com
(11) IIUW WILL LLL LUNCULILILLIOLI ULIOIULIJ CHILLS U U LIOL 3 Two Two Element X And Y Have Atomic Number 12 And 16 Respectively The element x has atomic number 12 and element y has atomic number 16. • electronic configuration of x : The electronic configuration of x will be 2, 8, 2 and that of y will. To which period of the modern periodic table do these two elements belong? What type of bond will be formed between them and. Both element. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From periodictable.me
Printable Periodic Table With Atomic Number Two Element X And Y Have Atomic Number 12 And 16 Respectively Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. • electronic configuration of x : • electronic configuration of y : To which period of the modern periodic table do. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.toppr.com
Two elements X and Y have atomic weights of 14 and 16. They form a Two Element X And Y Have Atomic Number 12 And 16 Respectively Two elements x and y have atomic numbers 12 and 16 respectively. Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. Electronic configuration of element 'y' atomic number. • electronic configuration of x : The. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From periodictable.me
Printable Periodic table with atomic number Dynamic Periodic Table of Two Element X And Y Have Atomic Number 12 And 16 Respectively Two elements x and y have atomic numbers 12 and 16 respectively. • electronic configuration of x : • electronic configuration of y : Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. To which period of the modern periodic table do these two elements belong? To which period of the modern. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.toppr.com
(20. Two elements X and Y have 6 and 7 electrons in their N and M Two Element X And Y Have Atomic Number 12 And 16 Respectively The element x has atomic number 12 and element y has atomic number 16. To which period of the modern periodic table do these two elements belong? Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. Magnesium being the metal will tend to lose an. Electronic configuration of element x, atomic number 12=2;8;2. Electronic configuration of element. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From elchoroukhost.net
Periodic Table Of The Elements Definition Biology Elcho Table Two Element X And Y Have Atomic Number 12 And 16 Respectively Two elements x and y have atomic numbers 12 and 16 respectively. The electronic configuration of x will be 2, 8, 2 and that of y will. Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. To which period of the modern periodic table do these two elements belong? Both element x (magnesium) and element y (sulfur),. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples Two Element X And Y Have Atomic Number 12 And 16 Respectively Electronic configuration of element 'y' atomic number. To which period of the modern periodic table do these two elements belong? To which period of the modern periodic table do these two elements belong? Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. What type of bond will. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.toppr.com
7. Two elements X and Y have atomic number 11 and 16 respectively. (a Two Element X And Y Have Atomic Number 12 And 16 Respectively Electronic configuration of element 'y' atomic number. Magnesium being the metal will tend to lose an. • electronic configuration of y : Electronic configuration of element x, atomic number 12=2;8;2. The electronic configuration of x will be 2, 8, 2 and that of y will. Two elements x and y have atomic numbers 12 and 16 respectively. Two elements $$x$$. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name Two Element X And Y Have Atomic Number 12 And 16 Respectively To which period of the modern periodic table do these two elements belong? The element x has atomic number 12 and element y has atomic number 16. Two elements x and y have atomic numbers 12 and 16 respectively. Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name Two Element X And Y Have Atomic Number 12 And 16 Respectively Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. Electronic configuration of element 'y' atomic number. To which period of the modern periodic table do these two elements belong? Magnesium being the metal will tend to lose an. What type of bond will be formed. • electronic configuration of x : Two elements x and y have. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From freehomedelivery.net
Periodic Table Periodic Table Of Elements, Table Of Elements, Modern Two Element X And Y Have Atomic Number 12 And 16 Respectively • electronic configuration of y : • electronic configuration of x : Electronic configuration of element 'y' atomic number. To which period of the modern periodic table do these two elements belong? Two elements x and y have atomic numbers 12 and 16 respectively. Magnesium being the metal will tend to lose an. What type of bond will be formed. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.toppr.com
Two elements X and Y have atomic number 12 and 16 respectively. To Two Element X And Y Have Atomic Number 12 And 16 Respectively Electronic configuration of element x, atomic number 12=2;8;2. Magnesium being the metal will tend to lose an. To which period of the modern periodic table do these two elements belong? • electronic configuration of y : The element x has atomic number 12 and element y has atomic number 16. To which period of the modern periodic table do these. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From sciencenotes.org
List of Elements By Atomic Number Two Element X And Y Have Atomic Number 12 And 16 Respectively The electronic configuration of x will be 2, 8, 2 and that of y will. Electronic configuration of element 'y' atomic number. Magnesium being the metal will tend to lose an. What type of bond will be formed between them and. What type of bond will be formed. To which period of the modern periodic table do these two elements. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From www.geeksforgeeks.org
Atomic Number of Elements Two Element X And Y Have Atomic Number 12 And 16 Respectively What type of bond will be formed between them and. • electronic configuration of y : Upon analysis, we find that the atomic numbers 12 and 16 belong to magnesium and sulfur respectively. The element x has atomic number 12 and element y has atomic number 16. Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. Electronic. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From ar.inspiredpencil.com
Printable Periodic Table With Names Of Elements And Atomic Numbers Two Element X And Y Have Atomic Number 12 And 16 Respectively Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong to the third period of the. The electronic configuration of x will be 2, 8, 2 and that of y will. To which period of the modern periodic table do these two elements belong? The element x has atomic number 12 and element y. Two Element X And Y Have Atomic Number 12 And 16 Respectively.
From utedzz.blogspot.com
Periodic Table Showing Mass Number And Atomic Number Periodic Table Two Element X And Y Have Atomic Number 12 And 16 Respectively Two elements $$x$$ and $$y$$ have atomic number $$12$$ and $$16$$ respectively. Two elements x and y have atomic numbers 12 and 16 respectively. Electronic configuration of element 'y' atomic number. To which period of the modern periodic table do these two elements belong? Both element x (magnesium) and element y (sulfur), with atomic numbers 12 and 16 respectively, belong. Two Element X And Y Have Atomic Number 12 And 16 Respectively.