What Is A Good Buffer . The ratio of acid and conjugate base required to give the correct ph. Buffers are often used in research on reactions involving enzymes. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A good buffer is chemically stable enough to resist degradation that enzymes could cause. Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. This characteristic makes buffers important in biological and. You will need to consider two factors: Weak bases and their salts are better as buffers for phs greater than 7. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or. In general, weak acids and their salts are better as buffers for phs less than 7; Buffer capacity depends upon the absolute quantities of. The amount of buffering required; Calculate the ph of a buffer before and after the addition of added acid or base.
from studylib.net
A good buffer is chemically stable enough to resist degradation that enzymes could cause. Calculate the ph of a buffer before and after the addition of added acid or base. The amount of buffering required; You will need to consider two factors: Weak bases and their salts are better as buffers for phs greater than 7. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The ratio of acid and conjugate base required to give the correct ph. Buffer capacity depends upon the absolute quantities of. It is able to neutralize small amounts of added acid or. This characteristic makes buffers important in biological and.
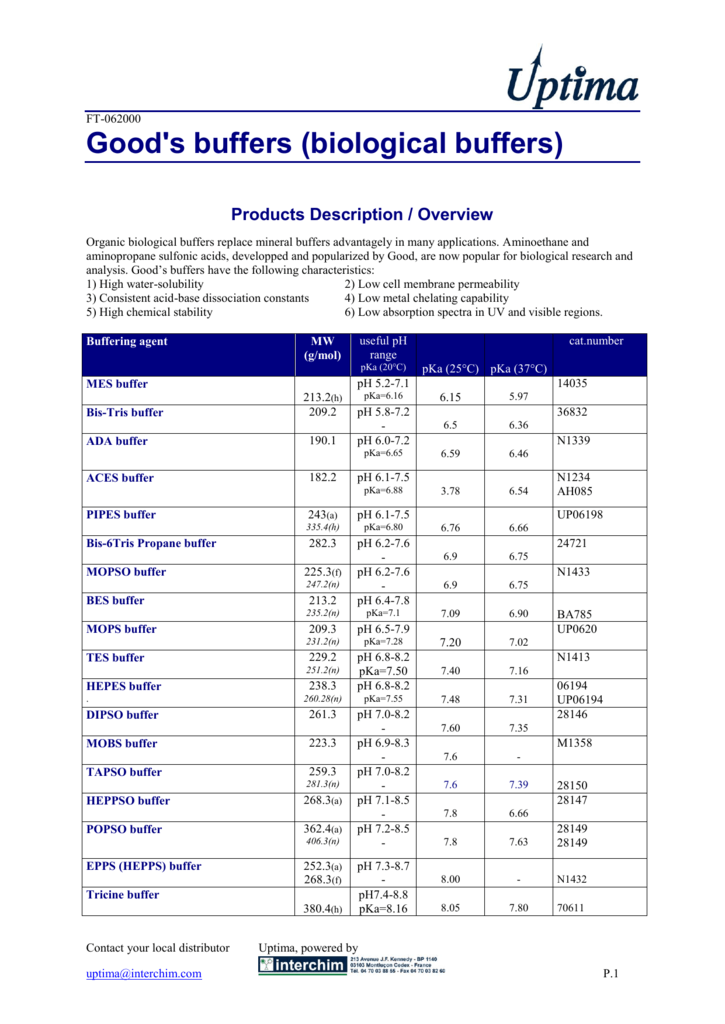
Good's buffers (biological buffers)
What Is A Good Buffer You will need to consider two factors: It is able to neutralize small amounts of added acid or. A good buffer is chemically stable enough to resist degradation that enzymes could cause. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In general, weak acids and their salts are better as buffers for phs less than 7; Weak bases and their salts are better as buffers for phs greater than 7. You will need to consider two factors: The ratio of acid and conjugate base required to give the correct ph. The amount of buffering required; A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are often used in research on reactions involving enzymes. Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. Buffer capacity depends upon the absolute quantities of. This characteristic makes buffers important in biological and. Calculate the ph of a buffer before and after the addition of added acid or base.
From hillalifeller.blogspot.com
What Makes A Good Buffer System Hill Alifeller What Is A Good Buffer A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In general, weak acids and their salts are better as buffers for phs less than 7; The amount of buffering required; This characteristic makes buffers important in biological and. Use the total buffer concentration and ph desired to. What Is A Good Buffer.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide What Is A Good Buffer This characteristic makes buffers important in biological and. Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. Weak bases and their salts are better as buffers for phs greater than 7. In general, weak acids and their salts are better as buffers for phs less than 7; The. What Is A Good Buffer.
From slideplayer.com
Faculty of medicine, U of D ppt download What Is A Good Buffer A good buffer is chemically stable enough to resist degradation that enzymes could cause. The amount of buffering required; Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. The ratio of acid and conjugate base required to give the correct ph. You will need to consider two factors:. What Is A Good Buffer.
From www.researchgate.net
Analysis of Good's buffer species MES and MOPS. (a) Chemical structure What Is A Good Buffer A good buffer is chemically stable enough to resist degradation that enzymes could cause. Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. In general, weak acids and their salts are better as buffers for phs less than 7; You will need to consider two factors: Calculate the. What Is A Good Buffer.
From www.slideshare.net
Buffer system What Is A Good Buffer Calculate the ph of a buffer before and after the addition of added acid or base. The ratio of acid and conjugate base required to give the correct ph. This characteristic makes buffers important in biological and. Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. It is. What Is A Good Buffer.
From www.youtube.com
Buffer capacity; making a buffer with a given pH value YouTube What Is A Good Buffer A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The amount of buffering required; It is able to neutralize small amounts of added acid or. The ratio of acid and conjugate base required to give the correct ph. Buffer capacity depends upon the absolute quantities of. A. What Is A Good Buffer.
From www.lookfordiagnosis.com
Buffers What Is A Good Buffer This characteristic makes buffers important in biological and. A good buffer is chemically stable enough to resist degradation that enzymes could cause. Calculate the ph of a buffer before and after the addition of added acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. What Is A Good Buffer.
From www.youtube.com
Buffer range and capacity YouTube What Is A Good Buffer The ratio of acid and conjugate base required to give the correct ph. It is able to neutralize small amounts of added acid or. This characteristic makes buffers important in biological and. You will need to consider two factors: In general, weak acids and their salts are better as buffers for phs less than 7; Use the total buffer concentration. What Is A Good Buffer.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Is A Good Buffer You will need to consider two factors: Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. Buffer capacity depends upon the absolute quantities of. Calculate the ph of a buffer before and after the addition of added acid or base. In general, weak acids and their salts are. What Is A Good Buffer.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Is A Good Buffer Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. In general, weak acids and their salts are better as buffers for phs less than 7; Buffer capacity depends upon the absolute quantities of. A buffer is a solution that can resist ph change upon the addition of an. What Is A Good Buffer.
From study.com
Buffer System in Chemistry Definition & Overview Video & Lesson What Is A Good Buffer It is able to neutralize small amounts of added acid or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and. A good buffer is chemically stable enough to resist degradation that enzymes could cause. A buffer is a solution that maintains the. What Is A Good Buffer.
From www.slideshare.net
Ch 18 buffers What Is A Good Buffer The amount of buffering required; This characteristic makes buffers important in biological and. Buffers are often used in research on reactions involving enzymes. In general, weak acids and their salts are better as buffers for phs less than 7; A good buffer is chemically stable enough to resist degradation that enzymes could cause. A buffer is a solution that maintains. What Is A Good Buffer.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog What Is A Good Buffer Calculate the ph of a buffer before and after the addition of added acid or base. This characteristic makes buffers important in biological and. The ratio of acid and conjugate base required to give the correct ph. Buffers are often used in research on reactions involving enzymes. Use the total buffer concentration and ph desired to calculate the amounts of. What Is A Good Buffer.
From klaamuahq.blob.core.windows.net
What Is A Buffer Quizlet at James Holden blog What Is A Good Buffer It is able to neutralize small amounts of added acid or. Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. A good buffer is chemically stable enough to resist degradation that enzymes could cause. Buffer capacity depends upon the absolute quantities of. A buffer is a solution that. What Is A Good Buffer.
From www.researchgate.net
Structures of Good's buffers. Long T 1 nuclei are indicated in red. The What Is A Good Buffer A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The amount of buffering required; A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are often used in research on reactions involving enzymes. In general, weak acids. What Is A Good Buffer.
From d2bygg93vzx8mv.cloudfront.net
同仁化学 Good's Buffer シリーズ|【ライフサイエンス】製品情報|試薬富士フイルム和光純薬 What Is A Good Buffer In general, weak acids and their salts are better as buffers for phs less than 7; It is able to neutralize small amounts of added acid or. Weak bases and their salts are better as buffers for phs greater than 7. Calculate the ph of a buffer before and after the addition of added acid or base. The ratio of. What Is A Good Buffer.
From sciencing.com
Characteristics of Good Buffers Sciencing What Is A Good Buffer It is able to neutralize small amounts of added acid or. Weak bases and their salts are better as buffers for phs greater than 7. A good buffer is chemically stable enough to resist degradation that enzymes could cause. The ratio of acid and conjugate base required to give the correct ph. A buffer is a solution that can resist. What Is A Good Buffer.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Is A Good Buffer It is able to neutralize small amounts of added acid or. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In general, weak acids and their salts are better as buffers for phs less than 7; Weak bases and their salts are better as buffers for phs. What Is A Good Buffer.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint What Is A Good Buffer Buffers are often used in research on reactions involving enzymes. The ratio of acid and conjugate base required to give the correct ph. You will need to consider two factors: Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. A good buffer is chemically stable enough to resist. What Is A Good Buffer.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Good Buffer This characteristic makes buffers important in biological and. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The ratio of acid and conjugate base required to give the correct ph. It is able to neutralize small amounts of added acid or. A buffer is a solution that. What Is A Good Buffer.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Is A Good Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The ratio of acid and conjugate base required to give the correct ph. The amount of buffering required; A good buffer is chemically stable enough to resist degradation that enzymes could cause. Buffer capacity depends upon the absolute quantities of. This. What Is A Good Buffer.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Is A Good Buffer Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. Weak bases and their salts are better as buffers for phs greater than 7. Buffer capacity depends upon the absolute quantities of. The amount of buffering required; The ratio of acid and conjugate base required to give the correct. What Is A Good Buffer.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is A Good Buffer This characteristic makes buffers important in biological and. The ratio of acid and conjugate base required to give the correct ph. Calculate the ph of a buffer before and after the addition of added acid or base. It is able to neutralize small amounts of added acid or. You will need to consider two factors: The amount of buffering required;. What Is A Good Buffer.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog What Is A Good Buffer Buffer capacity depends upon the absolute quantities of. It is able to neutralize small amounts of added acid or. Buffers are often used in research on reactions involving enzymes. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. You will need to consider two factors: Use the. What Is A Good Buffer.
From www.slideserve.com
PPT Novel Aminomethanesulfonate Buffers for Biological Buffering in What Is A Good Buffer Buffers are often used in research on reactions involving enzymes. Weak bases and their salts are better as buffers for phs greater than 7. Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. Calculate the ph of a buffer before and after the addition of added acid or. What Is A Good Buffer.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Is A Good Buffer The amount of buffering required; This characteristic makes buffers important in biological and. The ratio of acid and conjugate base required to give the correct ph. Weak bases and their salts are better as buffers for phs greater than 7. Calculate the ph of a buffer before and after the addition of added acid or base. Use the total buffer. What Is A Good Buffer.
From studyloadstones.z21.web.core.windows.net
Why Are Buffers Important What Is A Good Buffer Buffers are often used in research on reactions involving enzymes. You will need to consider two factors: It is able to neutralize small amounts of added acid or. The amount of buffering required; A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The ratio of acid and conjugate base required. What Is A Good Buffer.
From studylib.net
Good's buffers (biological buffers) What Is A Good Buffer Weak bases and their salts are better as buffers for phs greater than 7. This characteristic makes buffers important in biological and. Buffers are often used in research on reactions involving enzymes. The ratio of acid and conjugate base required to give the correct ph. Use the total buffer concentration and ph desired to calculate the amounts of acid and. What Is A Good Buffer.
From www.slideserve.com
PPT Novel Aminomethanesulfonate Buffers for Biological Buffering in What Is A Good Buffer Calculate the ph of a buffer before and after the addition of added acid or base. Buffer capacity depends upon the absolute quantities of. It is able to neutralize small amounts of added acid or. Buffers are often used in research on reactions involving enzymes. This characteristic makes buffers important in biological and. A buffer is a solution that can. What Is A Good Buffer.
From www.youtube.com
BUFFER SOLUTIONS HOW TO MAKE BUFFER ? TYPES OF BUFFER HOW BUFFERS What Is A Good Buffer Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids. What Is A Good Buffer.
From studyloadstones.z21.web.core.windows.net
Why Are Buffers Important What Is A Good Buffer A good buffer is chemically stable enough to resist degradation that enzymes could cause. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. The amount of. What Is A Good Buffer.
From www.pinterest.com
Pin on Download What Is A Good Buffer The amount of buffering required; A good buffer is chemically stable enough to resist degradation that enzymes could cause. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The ratio of acid and conjugate base required to give the correct ph. Use the total buffer concentration and. What Is A Good Buffer.
From chemistryguru.com.sg
What is a Buffer Solution? What Is A Good Buffer The amount of buffering required; Buffer capacity depends upon the absolute quantities of. This characteristic makes buffers important in biological and. The ratio of acid and conjugate base required to give the correct ph. Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. You will need to consider. What Is A Good Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID2453987 What Is A Good Buffer Use the total buffer concentration and ph desired to calculate the amounts of acid and base needed to create the buffer. A good buffer is chemically stable enough to resist degradation that enzymes could cause. The ratio of acid and conjugate base required to give the correct ph. The amount of buffering required; Weak bases and their salts are better. What Is A Good Buffer.
From www.promegaconnections.com
What Makes a "Good" Buffer? What Is A Good Buffer The ratio of acid and conjugate base required to give the correct ph. You will need to consider two factors: Buffer capacity depends upon the absolute quantities of. The amount of buffering required; Weak bases and their salts are better as buffers for phs greater than 7. Calculate the ph of a buffer before and after the addition of added. What Is A Good Buffer.