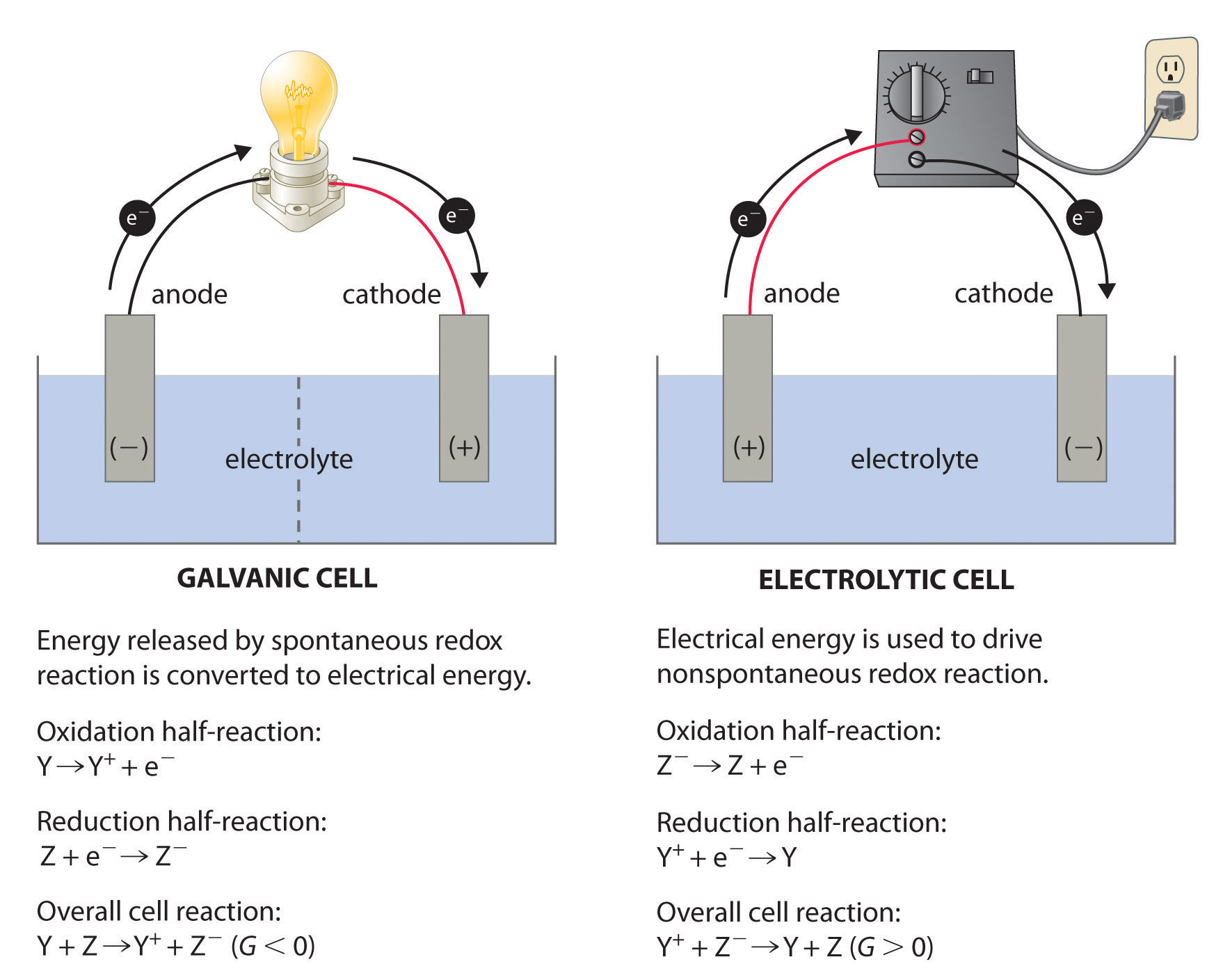
What Is An Electrochemical Battery . Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. A battery consists of electrochemical cells that convert stored chemical. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical reaction, heat generation, and.
from chem.libretexts.org
Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical reaction, heat generation, and. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. A battery consists of electrochemical cells that convert stored chemical.
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts
What Is An Electrochemical Battery In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical reaction, heat generation, and. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. A battery consists of electrochemical cells that convert stored chemical. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy.
From www.electricity-magnetism.org
How Batteries Work Basic Principle Electricity What Is An Electrochemical Battery In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. A battery consists of electrochemical. What Is An Electrochemical Battery.
From electrochemistryresources.com
Batteries / Electrochemical Cells What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Electrochemical reaction, heat generation, and. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. A battery consists of electrochemical cells that convert stored chemical. Batteries consist of. What Is An Electrochemical Battery.
From www.electricity-magnetism.org
How Batteries Work Basic Principle Electricity What Is An Electrochemical Battery Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current.. What Is An Electrochemical Battery.
From blog.strive2thrive.earth
Electrochemical Batteries A Solution for Energy Wastage? THRIVE blog What Is An Electrochemical Battery Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. A battery consists. What Is An Electrochemical Battery.
From www.researchgate.net
Fundamental design of electrochemical secondary battery cell [2] Download Scientific Diagram What Is An Electrochemical Battery Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. A battery consists of electrochemical cells that convert stored chemical. Electrochemical reaction, heat generation, and. Batteries consist of one or more. What Is An Electrochemical Battery.
From www.livescience.com
How Do Batteries Work? Live Science What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in. What Is An Electrochemical Battery.
From www.thoughtco.com
Electrochemical Cell Definition What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. A battery consists of electrochemical cells that convert stored chemical. Electrochemical reaction, heat generation, and. In a battery (also known as a galvanic cell), current is produced when electrons. What Is An Electrochemical Battery.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is An Electrochemical Battery A battery consists of electrochemical cells that convert stored chemical. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Electrochemical reaction, heat generation, and. In a battery (also known as a galvanic cell), current is produced when electrons. What Is An Electrochemical Battery.
From fixcarbattery.blogspot.com
Recondition Car battery Magnesium copper water battery What Is An Electrochemical Battery In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. A battery consists of electrochemical cells that convert stored chemical. Electrochemical batteries convert. What Is An Electrochemical Battery.
From saylordotorg.github.io
Electrochemistry What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. A battery consists of electrochemical cells that convert stored chemical. Electrochemical reaction, heat generation, and. Batteries consist of one or more. What Is An Electrochemical Battery.
From studylib.net
Electrochemical Cells (Batteries) What Is An Electrochemical Battery Electrochemical reaction, heat generation, and. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a.. What Is An Electrochemical Battery.
From classnotes.org.in
Batteries Chemistry, Class 12, Electro Chemistry What Is An Electrochemical Battery In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy.. What Is An Electrochemical Battery.
From www.pinterest.com
Electrochemistry Electrochemistry, Chemistry lessons, Chemistry experiments What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy.. What Is An Electrochemical Battery.
From saylordotorg.github.io
Electrochemistry What Is An Electrochemical Battery Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. A battery consists of electrochemical cells that convert stored chemical. Electrochemical reaction, heat generation, and. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit. What Is An Electrochemical Battery.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is An Electrochemical Battery Electrochemical reaction, heat generation, and. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the. What Is An Electrochemical Battery.
From www.researchgate.net
4 Schematic of the electrochemical discharging process in the... Download Scientific Diagram What Is An Electrochemical Battery In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. A battery consists of electrochemical cells that convert stored chemical. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Electrochemical reaction, any process either caused or accompanied. What Is An Electrochemical Battery.
From drexel.edu
A Unified Theory of Electrochemical Energy Storage Bridging Batteries and Supercapacitors What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a.. What Is An Electrochemical Battery.
From www.sliderbase.com
Electrochemical Terminology What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical reaction, heat generation, and. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons. What Is An Electrochemical Battery.
From www.britannica.com
Battery Composition, Types, & Uses Britannica What Is An Electrochemical Battery Electrochemical reaction, heat generation, and. A battery consists of electrochemical cells that convert stored chemical. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical. What Is An Electrochemical Battery.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. A battery consists of electrochemical cells that convert stored chemical. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit. What Is An Electrochemical Battery.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. A battery consists of electrochemical cells that convert stored chemical. Electrochemical reaction, heat generation, and. In a battery (also known as a galvanic cell), current is produced when electrons. What Is An Electrochemical Battery.
From cpb.iphy.ac.cn
Brief overview of electrochemical potential in lithium ion batteries What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. A battery consists of electrochemical cells that convert stored chemical. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit. What Is An Electrochemical Battery.
From www.researchgate.net
Schematic illustration of 1D electrochemical Liion battery model. Download Scientific Diagram What Is An Electrochemical Battery In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. A battery consists of electrochemical cells that convert stored chemical. Electrochemical reaction, any process either caused or accompanied. What Is An Electrochemical Battery.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts What Is An Electrochemical Battery Electrochemical reaction, heat generation, and. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical batteries convert chemical energy directly into electrical energy and provide. What Is An Electrochemical Battery.
From www.researchgate.net
Illustration of basic configuration of a lithium ion (rechargeable)... Download Scientific Diagram What Is An Electrochemical Battery Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. A battery consists of electrochemical cells that convert stored chemical. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. In a battery (also known as. What Is An Electrochemical Battery.
From www.researchgate.net
Schematic of lithium ion battery electrochemical model.... Download Scientific Diagram What Is An Electrochemical Battery Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical reaction, heat generation, and. A battery consists of electrochemical cells that convert stored chemical. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. In. What Is An Electrochemical Battery.
From wonderfulengineering.com
What Is An Electric Battery? What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy.. What Is An Electrochemical Battery.
From www.researchgate.net
8 Typical electrochemical battery cell. Download Scientific Diagram What Is An Electrochemical Battery Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one. What Is An Electrochemical Battery.
From www.electricity-magnetism.org
Composition of Battery Parts of Battery Electricity What Is An Electrochemical Battery Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. A battery consists of electrochemical cells that convert stored chemical. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most. What Is An Electrochemical Battery.
From www.slideserve.com
PPT Electrochemistry Batteries PowerPoint Presentation, free download ID5480148 What Is An Electrochemical Battery Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Electrochemical reaction, heat generation, and. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical reaction, any process either caused or accompanied by the passage of an. What Is An Electrochemical Battery.
From thepowerfacts.com
What is a Battery? (A to Z Explanation) The Power Facts What Is An Electrochemical Battery Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. A battery consists. What Is An Electrochemical Battery.
From www.researchgate.net
Schematic of the electrochemical battery model. Download Scientific Diagram What Is An Electrochemical Battery A battery consists of electrochemical cells that convert stored chemical. In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical batteries convert. What Is An Electrochemical Battery.
From ourfuture.energy
Salt Water Lamp OurFuture.Energy What Is An Electrochemical Battery In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. A battery consists of electrochemical cells that convert stored chemical. Electrochemical reaction, heat generation, and. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Electrochemical reaction, any. What Is An Electrochemical Battery.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is An Electrochemical Battery In a battery (also known as a galvanic cell), current is produced when electrons flow externally through the circuit from one substance to the another substance because of a. Electrochemical batteries convert chemical energy directly into electrical energy and provide dc current. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in. What Is An Electrochemical Battery.
From pubs.acs.org
How Batteries Store and Release Energy Explaining Basic Electrochemistry Journal of Chemical What Is An Electrochemical Battery Batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical energy. Electrochemical reaction, heat generation, and. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical batteries convert chemical energy directly into electrical energy and provide. What Is An Electrochemical Battery.