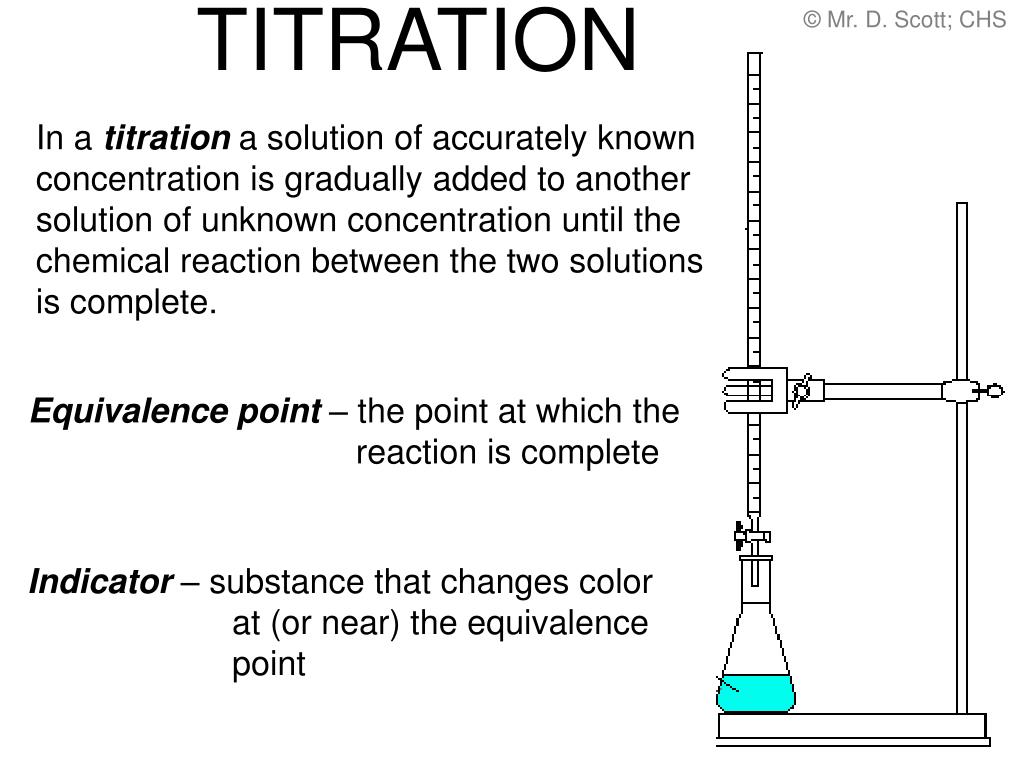
Titration Method Chemistry . Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The analyte (titrand) is the solution with an unknown molarity. A chemical reaction is set up between a known volume of a solution of unknown. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the titrant, until they. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. It is commonly used in analytical chemistry to determine the. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali.
from www.slideserve.com
A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the titrant, until they. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. It is commonly used in analytical chemistry to determine the. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The analyte (titrand) is the solution with an unknown molarity.
PPT TITRATION PowerPoint Presentation, free download ID1459481
Titration Method Chemistry A chemical reaction is set up between a known volume of a solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. The analyte (titrand) is the solution with an unknown molarity. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. It is commonly used in analytical chemistry to determine the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the titrant, until they. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A chemical reaction is set up between a known volume of a solution of unknown. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Method Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. The analyte (titrand) is the solution with an unknown molarity. The basic process involves adding a standard solution of. Titration Method Chemistry.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Method Chemistry A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the titrant, until they. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. It is commonly used in analytical chemistry to determine the. Titration is a procedure used in. Titration Method Chemistry.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry Titration Method Chemistry Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an. Titration Method Chemistry.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration Method Chemistry The analyte (titrand) is the solution with an unknown molarity. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. A titration is the process of determining the quantity. Titration Method Chemistry.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Method Chemistry The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. The analyte (titrand) is the solution with an unknown molarity. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a procedure used in chemistry. Titration Method Chemistry.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Method Chemistry Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. The analyte (titrand) is the solution with an unknown molarity. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a widely used technique to. Titration Method Chemistry.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Method Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Titration Method Chemistry.
From pubs.rsc.org
A convenient chemical titration method to measure M( ii )/M( iii Titration Method Chemistry It is commonly used in analytical chemistry to determine the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the titrant, until they. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is a widely. Titration Method Chemistry.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Titration Method Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is commonly used in analytical chemistry to determine the. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A chemical reaction. Titration Method Chemistry.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration Method Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration. Titration Method Chemistry.
From www.birmingham.ac.uk
Chemistry titrations University of Birmingham Titration Method Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Method Chemistry.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Method Chemistry A chemical reaction is set up between a known volume of a solution of unknown. It is commonly used in analytical chemistry to determine the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a procedure used in chemistry in order to determine. Titration Method Chemistry.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Method Chemistry A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the titrant, until they. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration. Titration Method Chemistry.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Method Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. It is commonly used in analytical chemistry to determine the. Titration is a procedure used in chemistry in order to. Titration Method Chemistry.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Method Chemistry A chemical reaction is set up between a known volume of a solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Titration is a procedure. Titration Method Chemistry.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Method Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. It is commonly used in analytical chemistry to determine the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Learn about the titration technique through. Titration Method Chemistry.
From mungfali.com
Acid Base Titration Calculation Titration Method Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A chemical reaction is set up between a known volume of a solution of unknown. It is commonly used in analytical chemistry to determine the. Learn about the titration technique through a practical experiment to determine the reacting volumes and. Titration Method Chemistry.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Method Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration. Titration Method Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Method Chemistry A chemical reaction is set up between a known volume of a solution of unknown. It is commonly used in analytical chemistry to determine the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The analyte (titrand) is the solution with an unknown molarity. A. Titration Method Chemistry.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration Method Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of. Titration Method Chemistry.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Method Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Method Chemistry.
From mungfali.com
Acid Base Titration Procedure Titration Method Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts. Titration Method Chemistry.
From bramblechemistry.weebly.com
4C6 Titration Titration Method Chemistry Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration Method Chemistry.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Method Chemistry Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A chemical reaction is set up between a known volume of a solution of. Titration Method Chemistry.
From www.science-revision.co.uk
Titrations Titration Method Chemistry Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is a laboratory technique used to precisely measure molar concentration of an unknown. Titration Method Chemistry.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Method Chemistry Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The basic process involves adding a standard solution of one reagent to a known. Titration Method Chemistry.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration Method Chemistry Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is a widely used technique to measure the amount of one substance present in. Titration Method Chemistry.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Method Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A chemical reaction is set up between a known volume of a solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly. Titration Method Chemistry.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Method Chemistry The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. The analyte (titrand) is the solution with an unknown molarity. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a. Titration Method Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Method Chemistry A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the titrant, until they. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is a widely used technique to measure the amount of one substance. Titration Method Chemistry.
From www.sliderbase.com
Titration Titration Method Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. It is commonly used in analytical chemistry to determine the. A titration is the process of. Titration Method Chemistry.
From mungfali.com
Acid Base Titration Procedure Titration Method Chemistry The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. Titration Method Chemistry.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Method Chemistry It is commonly used in analytical chemistry to determine the. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A chemical reaction is set up between a known volume of a solution of unknown. Titration is a procedure used in chemistry in order to determine the molarity of an. Titration Method Chemistry.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Method Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is commonly used in analytical chemistry to determine the. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A chemical reaction. Titration Method Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Method Chemistry Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the titrant, until they. It is commonly used in analytical chemistry to determine the. Learn about the titration technique through a. Titration Method Chemistry.