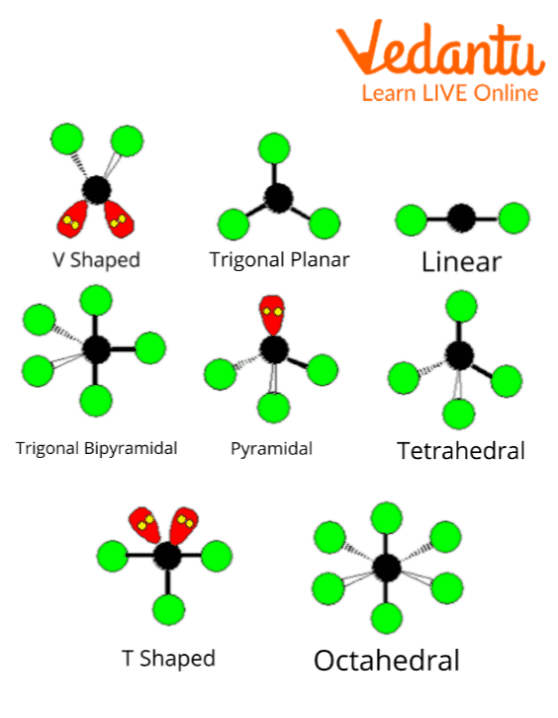
What Is True About Molecules . H2, n2, o2, f2, cl2, br2, and i2. The nucleus consists of protons and neutrons, of different numbers in different elements. Molecules are neutral and carry no. Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. When the atoms are from. Elements that exist as diatomic molecules: Molecules are made when two or more atoms chemically bond together. Elements that exist as polyatomic molecules: A molecule is a combination of two or more atoms that are held together by chemical bonds, such as covalent bonds and ionic bonds. Whenever two or more atoms combine, they form a molecule. It is the smallest unit of a chemical substance having all the properties of that substance. Atoms from different elements can combine. The cation (first part of the molecule) and anion (second part of the molecule) have very. Each atom carries a certain number of electrons that orbit around the nucleus. A molecule is two or more atoms bonded together to form a single chemical entity.
from www.vedantu.com
Elements that exist as polyatomic molecules: It is the smallest unit of a chemical substance having all the properties of that substance. When the atoms are from. A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. Elements that exist as diatomic molecules: Molecules are made when two or more atoms chemically bond together. Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. The cation (first part of the molecule) and anion (second part of the molecule) have very. Atoms from different elements can combine. The nucleus consists of protons and neutrons, of different numbers in different elements.
Facts About Molecules Learn Important Terms and Concepts
What Is True About Molecules The nucleus consists of protons and neutrons, of different numbers in different elements. Molecules are neutral and carry no. Each atom carries a certain number of electrons that orbit around the nucleus. A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. Molecules are made when two or more atoms chemically bond together. A molecule is the smallest unit of a compound that. Whenever two or more atoms combine, they form a molecule. Elements that exist as diatomic molecules: The nucleus consists of protons and neutrons, of different numbers in different elements. A molecule is a combination of two or more atoms that are held together by chemical bonds, such as covalent bonds and ionic bonds. A molecule is two or more atoms bonded together to form a single chemical entity. Elements that exist as polyatomic molecules: Atoms from different elements can combine. H2, n2, o2, f2, cl2, br2, and i2. Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. It is the smallest unit of a chemical substance having all the properties of that substance.
From sciencenotes.org
What Is a Molecule? Definition and Examples What Is True About Molecules The cation (first part of the molecule) and anion (second part of the molecule) have very. Elements that exist as diatomic molecules: When the atoms are from. Each atom carries a certain number of electrons that orbit around the nucleus. The nucleus consists of protons and neutrons, of different numbers in different elements. Atoms are the basic or fundamental units. What Is True About Molecules.
From www.thedailyeco.com
The Differences Between Atoms and Molecules Atom and Molecule What Is True About Molecules A molecule is two or more atoms bonded together to form a single chemical entity. Atoms from different elements can combine. Each atom carries a certain number of electrons that orbit around the nucleus. Whenever two or more atoms combine, they form a molecule. The nucleus consists of protons and neutrons, of different numbers in different elements. Elements that exist. What Is True About Molecules.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples What Is True About Molecules When the atoms are from. Elements that exist as polyatomic molecules: Atoms from different elements can combine. Each atom carries a certain number of electrons that orbit around the nucleus. A molecule is the smallest unit of a compound that. Molecules are made when two or more atoms chemically bond together. The nucleus consists of protons and neutrons, of different. What Is True About Molecules.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I What Is True About Molecules Molecules are neutral and carry no. A molecule is two or more atoms bonded together to form a single chemical entity. A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. Elements that exist as diatomic molecules: A molecule. What Is True About Molecules.
From www.sliderbase.com
Elements Presentation Chemistry What Is True About Molecules A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. Elements that exist as diatomic molecules: Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. The nucleus consists of. What Is True About Molecules.
From www.vedantu.com
Facts About Molecules Learn Important Terms and Concepts What Is True About Molecules Molecules are neutral and carry no. Atoms from different elements can combine. The cation (first part of the molecule) and anion (second part of the molecule) have very. A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. It. What Is True About Molecules.
From www.inkl.com
What do molecules look like? What Is True About Molecules Elements that exist as diatomic molecules: Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. It is the smallest. What Is True About Molecules.
From enthu.com
How are Molecules Formed? EnthuZiastic What Is True About Molecules Each atom carries a certain number of electrons that orbit around the nucleus. Molecules are made when two or more atoms chemically bond together. When the atoms are from. Molecules are neutral and carry no. Elements that exist as diatomic molecules: A molecule is two or more atoms bonded together to form a single chemical entity. H2, n2, o2, f2,. What Is True About Molecules.
From www.slideserve.com
PPT Atoms and Molecules PowerPoint Presentation, free download ID What Is True About Molecules When the atoms are from. Elements that exist as polyatomic molecules: Whenever two or more atoms combine, they form a molecule. A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. A molecule is two or more atoms bonded. What Is True About Molecules.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation What Is True About Molecules A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. H2, n2, o2, f2, cl2, br2, and i2. Whenever two or more atoms combine, they form a molecule. The cation (first part of the molecule) and anion (second part. What Is True About Molecules.
From brainly.com
Which of the following statements about molecules is true? A. All What Is True About Molecules It is the smallest unit of a chemical substance having all the properties of that substance. A molecule is a combination of two or more atoms that are held together by chemical bonds, such as covalent bonds and ionic bonds. Elements that exist as diatomic molecules: Atoms are the basic or fundamental units of matter that rarely exist independently but. What Is True About Molecules.
From www.vedantu.com
Facts About Molecules Learn Important Terms and Concepts What Is True About Molecules Molecules are made when two or more atoms chemically bond together. A molecule is the smallest unit of a compound that. The cation (first part of the molecule) and anion (second part of the molecule) have very. Whenever two or more atoms combine, they form a molecule. Molecules are neutral and carry no. Atoms are the basic or fundamental units. What Is True About Molecules.
From study.com
Molecules Definition Lesson for Kids Lesson What Is True About Molecules Whenever two or more atoms combine, they form a molecule. The cation (first part of the molecule) and anion (second part of the molecule) have very. A molecule is two or more atoms bonded together to form a single chemical entity. It is the smallest unit of a chemical substance having all the properties of that substance. Molecules are neutral. What Is True About Molecules.
From www.pinterest.com
Molecule Easy Science Teaching chemistry, Molecules, Easy science What Is True About Molecules H2, n2, o2, f2, cl2, br2, and i2. Each atom carries a certain number of electrons that orbit around the nucleus. Elements that exist as polyatomic molecules: When the atoms are from. Elements that exist as diatomic molecules: A molecule is a combination of two or more atoms that are held together by chemical bonds, such as covalent bonds and. What Is True About Molecules.
From www.youtube.com
4 Biological Molecules Structure and Their Function A quick guide What Is True About Molecules Molecules are neutral and carry no. Atoms from different elements can combine. It is the smallest unit of a chemical substance having all the properties of that substance. When the atoms are from. The nucleus consists of protons and neutrons, of different numbers in different elements. A molecule is two or more atoms bonded together to form a single chemical. What Is True About Molecules.
From www.sciencefacts.net
Molecule Definition, Examples, Facts & Diagram What Is True About Molecules Molecules are made when two or more atoms chemically bond together. Elements that exist as polyatomic molecules: Elements that exist as diatomic molecules: H2, n2, o2, f2, cl2, br2, and i2. A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties. What Is True About Molecules.
From scholarsark.com
What Is A Molecule? Scholars Ark What Is True About Molecules Whenever two or more atoms combine, they form a molecule. It is the smallest unit of a chemical substance having all the properties of that substance. H2, n2, o2, f2, cl2, br2, and i2. Elements that exist as diatomic molecules: Atoms from different elements can combine. A molecule is two or more atoms that form chemical bonds with each other,. What Is True About Molecules.
From www.britannica.com
Chemical compound Definition, Examples, & Types Britannica What Is True About Molecules Elements that exist as polyatomic molecules: Molecules are neutral and carry no. The nucleus consists of protons and neutrons, of different numbers in different elements. Molecules are made when two or more atoms chemically bond together. Each atom carries a certain number of electrons that orbit around the nucleus. A molecule is a combination of two or more atoms that. What Is True About Molecules.
From scienceatyourdoorstep.com
Types of Atoms Science at Your Doorstep What Is True About Molecules Whenever two or more atoms combine, they form a molecule. H2, n2, o2, f2, cl2, br2, and i2. Elements that exist as diatomic molecules: Atoms from different elements can combine. It is the smallest unit of a chemical substance having all the properties of that substance. The nucleus consists of protons and neutrons, of different numbers in different elements. A. What Is True About Molecules.
From www.thoughtco.com
Definition and Examples of a Molecule What Is True About Molecules Whenever two or more atoms combine, they form a molecule. Molecules are neutral and carry no. Each atom carries a certain number of electrons that orbit around the nucleus. Elements that exist as diatomic molecules: A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the. What Is True About Molecules.
From www.thoughtco.com
Definition and Examples of a Molecule What Is True About Molecules Each atom carries a certain number of electrons that orbit around the nucleus. A molecule is two or more atoms bonded together to form a single chemical entity. Elements that exist as diatomic molecules: The cation (first part of the molecule) and anion (second part of the molecule) have very. Whenever two or more atoms combine, they form a molecule.. What Is True About Molecules.
From www.snexplores.org
Scientists Say Molecule What Is True About Molecules A molecule is two or more atoms bonded together to form a single chemical entity. Elements that exist as polyatomic molecules: A molecule is a combination of two or more atoms that are held together by chemical bonds, such as covalent bonds and ionic bonds. It is the smallest unit of a chemical substance having all the properties of that. What Is True About Molecules.
From www.chegg.com
Solved Question 4 What is true about atoms and molecules? What Is True About Molecules A molecule is two or more atoms bonded together to form a single chemical entity. The nucleus consists of protons and neutrons, of different numbers in different elements. Elements that exist as diatomic molecules: Whenever two or more atoms combine, they form a molecule. The cation (first part of the molecule) and anion (second part of the molecule) have very.. What Is True About Molecules.
From www.youtube.com
When one DNA molecule is replicated what is true about the second What Is True About Molecules Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. A molecule is two or more atoms bonded together to form a single chemical entity. A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical. What Is True About Molecules.
From study.com
Simple Molecules Examples & Explanation Video & Lesson Transcript What Is True About Molecules Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. Whenever two or more atoms combine, they form a molecule. A molecule is the smallest unit of a compound that. The nucleus consists of protons and neutrons, of different numbers in different elements. Atoms from different elements can combine. Molecules are. What Is True About Molecules.
From www.youtube.com
How Molecules are formed ? Animated Lesson for kids YouTube What Is True About Molecules Molecules are made when two or more atoms chemically bond together. When the atoms are from. Each atom carries a certain number of electrons that orbit around the nucleus. Whenever two or more atoms combine, they form a molecule. H2, n2, o2, f2, cl2, br2, and i2. A molecule is a combination of two or more atoms that are held. What Is True About Molecules.
From www.sciencefacts.net
Molecule Definition, Examples, Facts & Diagram What Is True About Molecules Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. The cation (first part of the molecule) and anion (second part of the molecule) have very. Molecules are neutral and carry no. A molecule is two or more atoms bonded together to form a single chemical entity. A molecule is the. What Is True About Molecules.
From www.vedantu.com
Facts About Molecules Learn Important Terms and Concepts What Is True About Molecules Each atom carries a certain number of electrons that orbit around the nucleus. Elements that exist as polyatomic molecules: When the atoms are from. The cation (first part of the molecule) and anion (second part of the molecule) have very. A molecule is a combination of two or more atoms that are held together by chemical bonds, such as covalent. What Is True About Molecules.
From www.numerade.com
SOLVEDQUESTION 4 Which statement Is true about the molecules below OH What Is True About Molecules Elements that exist as diatomic molecules: A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. It is the smallest unit of a chemical substance having all the properties of that substance. Whenever two or more atoms combine, they. What Is True About Molecules.
From www.worksheetsplanet.com
What is a Molecule Meaning & Definition What Is True About Molecules The nucleus consists of protons and neutrons, of different numbers in different elements. A molecule is a combination of two or more atoms that are held together by chemical bonds, such as covalent bonds and ionic bonds. A molecule is two or more atoms bonded together to form a single chemical entity. Atoms are the basic or fundamental units of. What Is True About Molecules.
From www.yourdictionary.com
Basic Difference Between an Atom and a Molecule YourDictionary What Is True About Molecules H2, n2, o2, f2, cl2, br2, and i2. The nucleus consists of protons and neutrons, of different numbers in different elements. Atoms from different elements can combine. When the atoms are from. Elements that exist as diatomic molecules: Each atom carries a certain number of electrons that orbit around the nucleus. Atoms are the basic or fundamental units of matter. What Is True About Molecules.
From www.youtube.com
Molecule vs Compound Examples and Practice YouTube What Is True About Molecules Atoms from different elements can combine. The nucleus consists of protons and neutrons, of different numbers in different elements. Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances. When the atoms are from. It is the smallest unit of a chemical substance having all the properties of that substance. Molecules. What Is True About Molecules.
From www.slideserve.com
PPT Atoms and Molecules PowerPoint Presentation, free download ID What Is True About Molecules Each atom carries a certain number of electrons that orbit around the nucleus. A molecule is two or more atoms bonded together to form a single chemical entity. The nucleus consists of protons and neutrons, of different numbers in different elements. Atoms are the basic or fundamental units of matter that rarely exist independently but combine to form different substances.. What Is True About Molecules.
From www.slideserve.com
PPT Atoms Molecules Elements Compounds PowerPoint Presentation ID What Is True About Molecules A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. Elements that exist as polyatomic molecules: A molecule is two or more atoms bonded together to form a single chemical entity. Each atom carries a certain number of electrons. What Is True About Molecules.
From study.com
What are Atoms & Molecules? Definition & Differences Video & Lesson What Is True About Molecules A molecule is two or more atoms that form chemical bonds with each other, representing the smallest unit of a chemical compound with all the physical and chemical properties of the. The cation (first part of the molecule) and anion (second part of the molecule) have very. Molecules are made when two or more atoms chemically bond together. It is. What Is True About Molecules.