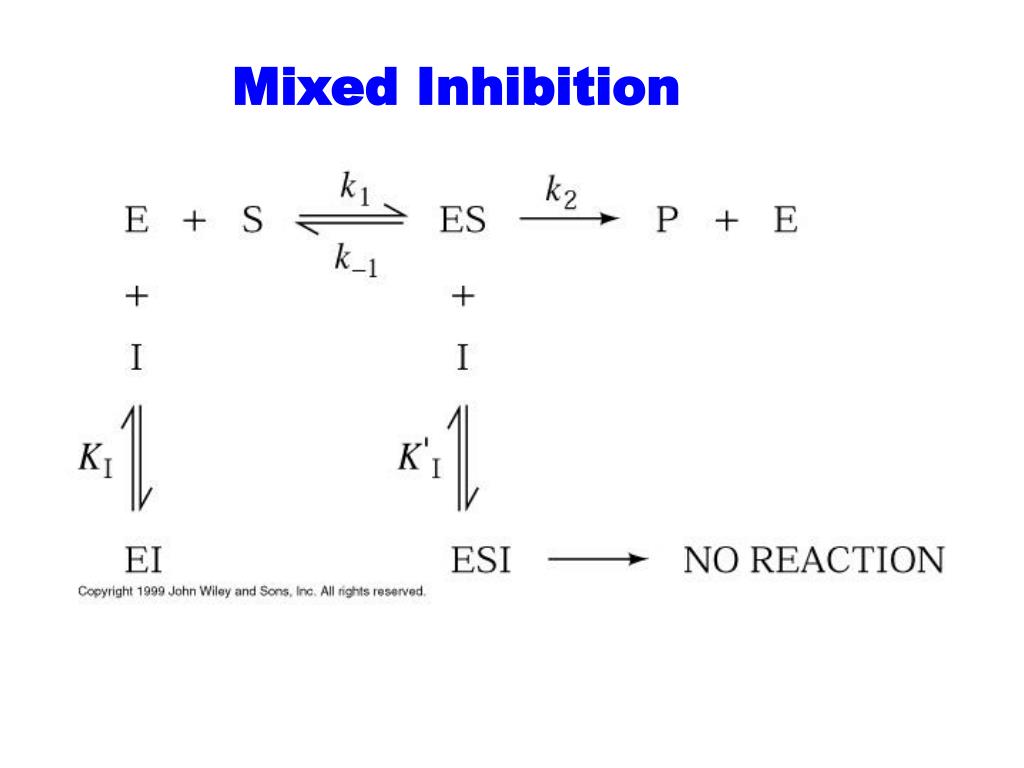
Mixed Inhibition Km And Vmax . Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Vmax is reached when all of the enzyme is in. Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Mixed inhibitors that act like competitive inhibitors by binding primarily. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly.
from www.slideserve.com
Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Mixed inhibitors that act like competitive inhibitors by binding primarily. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Vmax is reached when all of the enzyme is in. Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’.
PPT Enzyme PowerPoint Presentation, free download ID5086655
Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Vmax is reached when all of the enzyme is in. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Mixed inhibitors that act like competitive inhibitors by binding primarily.
From www.slideserve.com
PPT Chapter 12 Enzyme Inhibition, and Control PowerPoint Mixed Inhibition Km And Vmax Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Vmax is reached when all of the enzyme is in. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Vmax is the maximum velocity, or how fast the enzyme can go at. Mixed Inhibition Km And Vmax.
From www.lecturio.com
Enzyme Inhibition Concise Medical Knowledge Mixed Inhibition Km And Vmax Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Mixed inhibitors that act like competitive inhibitors by binding primarily. Vmax is reached when all of the enzyme is in. Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Most types of mixed inhibitors. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT תרגיל 1 PowerPoint Presentation, free download ID6238361 Mixed Inhibition Km And Vmax Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Probably the easiest type of enzyme inhibition to understand is competitive. Mixed Inhibition Km And Vmax.
From schoolbag.info
Biochemistry MCAT Biology and Biochemistry Mixed Inhibition Km And Vmax Mixed inhibitors that act like competitive inhibitors by binding primarily. Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax.. Mixed Inhibition Km And Vmax.
From www.numerade.com
SOLVED 4 /mixed inhibitor of an enzyme Increases Km Mixed Inhibition Km And Vmax Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Mixed and noncompetitive inhibition (as shown by the mechanism. Mixed Inhibition Km And Vmax.
From www.numerade.com
SOLVED Find below the reaction scheme for mixed inhibition What Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Enzyme activators lower km (the michaelis constant) and/or raise vmax. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID305372 Mixed Inhibition Km And Vmax Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Vmax is reached when all of the enzyme is in. Most types of mixed inhibitors have a preference for one or the other, which dictates the. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation ID305372 Mixed Inhibition Km And Vmax Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Vmax is reached when all of the enzyme is in. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition. Mixed Inhibition Km And Vmax.
From www.chegg.com
o An introduction to modes of enzyme inhibition four Mixed Inhibition Km And Vmax Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Mixed inhibitors that act like competitive inhibitors by binding primarily. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Vmax is reached when all of the enzyme is in. Enzyme activators lower km (the michaelis constant). Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Lecture Notes for Chapter 7 Enzyme and Inhibition Mixed Inhibition Km And Vmax Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Mixed inhibitors that act like competitive inhibitors by binding primarily. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that. Mixed Inhibition Km And Vmax.
From iubmb.onlinelibrary.wiley.com
When both Km and Vmax are altered, Is the enzyme inhibited or activated Mixed Inhibition Km And Vmax Mixed inhibitors that act like competitive inhibitors by binding primarily. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Vmax is the maximum velocity, or how fast the enzyme can. Mixed Inhibition Km And Vmax.
From www.numerade.com
SOLVED Find below the reaction scheme for mixed inhibition What Mixed Inhibition Km And Vmax Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax.. Mixed Inhibition Km And Vmax.
From faaiznarendra.blogspot.com
47+ how to calculate ki for competitive inhibition FaaizNarendra Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Vmax is reached when all of the enzyme is in. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition. Mixed Inhibition Km And Vmax.
From www.biologyexams4u.com
Biology Exams 4 U Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Mixed inhibitors that act like competitive inhibitors by. Mixed Inhibition Km And Vmax.
From www.youtube.com
Mixed inhibition and Non competitive inhibition YouTube Mixed Inhibition Km And Vmax Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Mixed inhibitors that act like competitive inhibitors by binding primarily. Vmax is reached when all of the enzyme is in. Mixed and noncompetitive inhibition (as shown. Mixed Inhibition Km And Vmax.
From studyonline.netlify.app
Non competitive inhibitor example Mixed Inhibition Km And Vmax Mixed inhibitors that act like competitive inhibitors by binding primarily. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Vmax is reached when all of the enzyme is in. Vmax. Mixed Inhibition Km And Vmax.
From alevelnotes.com
Enzyme Inhibitors A Level Notes Mixed Inhibition Km And Vmax Mixed inhibitors that act like competitive inhibitors by binding primarily. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Most types of mixed inhibitors have a preference for one or. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT LAB 3 Enzyme PowerPoint Presentation, free download ID Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Mixed and noncompetitive inhibition (as shown. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID305372 Mixed Inhibition Km And Vmax Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Mixed inhibitors that act like competitive inhibitors by binding primarily. Vmax is reached when all of the enzyme is in. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Thus, a. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID5086655 Mixed Inhibition Km And Vmax Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Thus, a competitive inhibitor does not. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT LECTURE 2 ENZYME PowerPoint Presentation, free download Mixed Inhibition Km And Vmax Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Thus,. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410 Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Mixed inhibitors that act like competitive inhibitors by binding primarily. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Most. Mixed Inhibition Km And Vmax.
From www.animalia-life.club
Mixed Inhibition Graph Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Most types of mixed inhibitors have a preference for one or the other,. Mixed Inhibition Km And Vmax.
From www.reddit.com
Can anyone explain Km and Vmax? I'm trying to memorize what happens to Mixed Inhibition Km And Vmax Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Probably the easiest type of enzyme inhibition to understand is. Mixed Inhibition Km And Vmax.
From www.youtube.com
Constants Km & Vmax YouTube Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Vmax is reached when all of the enzyme is in. Mixed inhibitors that act like competitive inhibitors by binding primarily. Thus, a competitive inhibitor. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Inhibition of enzyme activity PowerPoint Presentation, free Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Mixed inhibitors that act like competitive. Mixed Inhibition Km And Vmax.
From www.numerade.com
No inhibitor Competitive inhibitor Vmax = Vo Mixed inhibitor Answer Mixed Inhibition Km And Vmax Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Vmax is reached when all of the enzyme is in. Mixed inhibitors that act like competitive inhibitors by binding primarily. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Thus,. Mixed Inhibition Km And Vmax.
From hra.animalia-life.club
Mixed Inhibition Mixed Inhibition Km And Vmax Mixed inhibitors that act like competitive inhibitors by binding primarily. Vmax is reached when all of the enzyme is in. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax.. Mixed Inhibition Km And Vmax.
From www.animalia-life.club
Mixed Inhibition Graph Mixed Inhibition Km And Vmax Vmax is reached when all of the enzyme is in. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Mixed inhibitors that act like competitive inhibitors by binding primarily. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Thus, a competitive. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Lecture 7Enzyme InhibitionDrug Discovery PowerPoint Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Mixed inhibitors that act like competitive inhibitors by binding primarily. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax.. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Enzymes Basic Concepts and PowerPoint Presentation Mixed Inhibition Km And Vmax Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Mixed inhibitors that act like competitive. Mixed Inhibition Km And Vmax.
From courses.lumenlearning.com
Enzymes OpenStax Biology 2e Mixed Inhibition Km And Vmax Probably the easiest type of enzyme inhibition to understand is competitive inhibition and it is the one most commonly. Vmax is reached when all of the enzyme is in. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Vmax is the maximum velocity, or how fast the enzyme can go at. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Lecture Notes for Chapter 7 Enzyme and Inhibition Mixed Inhibition Km And Vmax Vmax is reached when all of the enzyme is in. Mixed inhibitors that act like competitive inhibitors by binding primarily. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Most types of mixed inhibitors have a preference for one or the other, which dictates the effect on km and vmax. Mixed and noncompetitive inhibition (as. Mixed Inhibition Km And Vmax.
From www.slideserve.com
PPT Lecture 16 PowerPoint Presentation, free download ID559678 Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Vmax is reached when all of the enzyme is in. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition in that the inhibitor. Most. Mixed Inhibition Km And Vmax.
From www.numerade.com
SOLVED An inhibitor raises the Km of an enzyme but does not change its Mixed Inhibition Km And Vmax Vmax is the maximum velocity, or how fast the enzyme can go at full ‘‘speed.’’. Vmax is reached when all of the enzyme is in. Thus, a competitive inhibitor does not affect the maximum activity (vmax) of an enzyme. Enzyme activators lower km (the michaelis constant) and/or raise vmax (the asymptotic reaction velocity at infinite substrate concentration); Probably the easiest. Mixed Inhibition Km And Vmax.