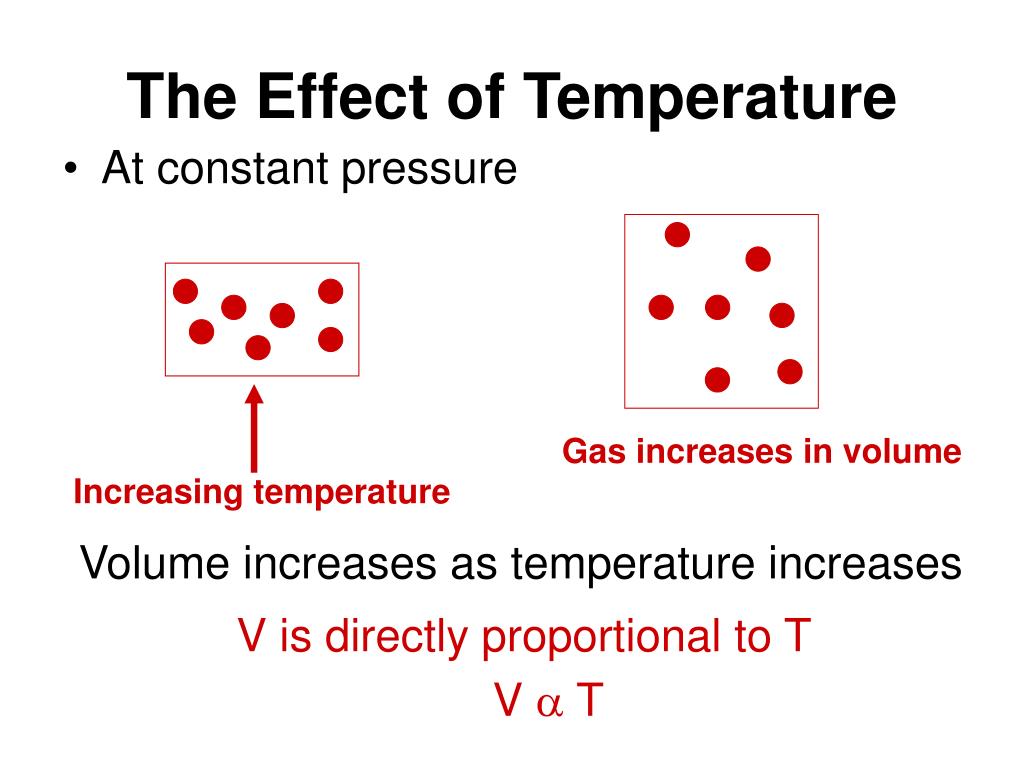
Does Increasing Pressure Increase Volume . As one decreases, the other increases. We know that pressure and volume are inversely related; If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. If you lower the pressure, volume increases. Where c is a constant. Pv=c p v = c. In fact, if the volume. In fact, if the volume. This relationship, known as boyle's law, is one of the cornerstones of. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In reality, most compression take place by reducing volume or. As the pressure on a gas increases, the volume of the gas decreases because the gas. Pressure is decreasing (from 2.44 atm. If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be.
from www.slideserve.com
Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In reality, most compression take place by reducing volume or. If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. Pv=c p v = c. In fact, if the volume. Pressure is decreasing (from 2.44 atm. The relationship between pressure and volume: As one decreases, the other increases. We know that pressure and volume are inversely related; This relationship, known as boyle's law, is one of the cornerstones of.
PPT The Ideal Gas Equation PowerPoint Presentation, free download
Does Increasing Pressure Increase Volume If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. If you lower the pressure, volume increases. If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. In reality, most compression take place by reducing volume or. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. We know that pressure and volume are inversely related; Pv=c p v = c. As the pressure on a gas increases, the volume of the gas decreases because the gas. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. The relationship between pressure and volume: Pressure is decreasing (from 2.44 atm. This relationship, known as boyle's law, is one of the cornerstones of. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. As one decreases, the other increases. Where c is a constant. In fact, if the volume.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial Does Increasing Pressure Increase Volume Pv=c p v = c. As one decreases, the other increases. In fact, if the volume. As the pressure on a gas increases, the volume of the gas decreases because the gas. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The relationship between pressure and volume:. Does Increasing Pressure Increase Volume.
From sciencenotes.org
Boyle's Law Definition, Formula, Example Does Increasing Pressure Increase Volume As the pressure on a gas increases, the volume of the gas decreases because the gas. The relationship between pressure and volume: In fact, if the volume. In fact, if the volume. We know that pressure and volume are inversely related; If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the. Does Increasing Pressure Increase Volume.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Does Increasing Pressure Increase Volume In fact, if the volume. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. If you lower the pressure, volume increases. If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. In reality, most compression take place. Does Increasing Pressure Increase Volume.
From www.pinterest.com
Boyles Law States that pressure increases when volume decreases, and Does Increasing Pressure Increase Volume If you lower the pressure, volume increases. Where c is a constant. In reality, most compression take place by reducing volume or. As one decreases, the other increases. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. We know that pressure and volume are inversely related; Decreasing the volume of. Does Increasing Pressure Increase Volume.
From ecgwaves.com
Ventricular PressureVolume Relationship Preload, Afterload, Stroke Does Increasing Pressure Increase Volume As one decreases, the other increases. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. If you lower the pressure, volume increases. In fact, if the volume. Pressure is decreasing (from 2.44 atm. Where c is a constant. As the pressure on a gas increases, the volume of the gas. Does Increasing Pressure Increase Volume.
From question.pandai.org
Pressure in Liquid Does Increasing Pressure Increase Volume In fact, if the volume. The relationship between pressure and volume: Where c is a constant. In reality, most compression take place by reducing volume or. As one decreases, the other increases. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. We know that pressure and volume are inversely related;. Does Increasing Pressure Increase Volume.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Increasing Pressure Increase Volume Where c is a constant. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. We know that pressure and volume are inversely related; Pv=c p v = c. If you lower the pressure, volume increases. If you had a way to increase pressure with no volume change, then yes, temperature. Does Increasing Pressure Increase Volume.
From masterconceptsinchemistry.com
As pressure increase the volume of gas decrease Does Increasing Pressure Increase Volume Pv=c p v = c. This relationship, known as boyle's law, is one of the cornerstones of. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In reality, most compression take place by reducing volume or. Pressure is decreasing (from 2.44 atm. As one decreases, the other increases. We know. Does Increasing Pressure Increase Volume.
From www.shalom-education.com
Volume and Pressure in Gases GCSE Physics Revision Does Increasing Pressure Increase Volume In fact, if the volume. In reality, most compression take place by reducing volume or. The relationship between pressure and volume: Pressure is decreasing (from 2.44 atm. We know that pressure and volume are inversely related; As one decreases, the other increases. Pv=c p v = c. Where c is a constant. If you had a way to increase pressure. Does Increasing Pressure Increase Volume.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Does Increasing Pressure Increase Volume Pressure is decreasing (from 2.44 atm. In reality, most compression take place by reducing volume or. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. As the pressure on a gas increases, the volume of the gas decreases because the gas. If you lower the pressure, volume increases. If you. Does Increasing Pressure Increase Volume.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Does Increasing Pressure Increase Volume The relationship between pressure and volume: This relationship, known as boyle's law, is one of the cornerstones of. As one decreases, the other increases. In fact, if the volume. Pressure is decreasing (from 2.44 atm. We know that pressure and volume are inversely related; Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will. Does Increasing Pressure Increase Volume.
From www.youtube.com
15.9 The Effect of a Volume Change on Equilibrium YouTube Does Increasing Pressure Increase Volume If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. This relationship, known as boyle's law, is one of the cornerstones of. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume. Where. Does Increasing Pressure Increase Volume.
From www.omniamfg.com
Understanding the PressureVolume Diagrams — Omnia MFG Does Increasing Pressure Increase Volume If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. As the pressure on a gas increases, the volume of the gas decreases because the gas. As one decreases, the other increases. In fact, if the volume. Decreasing the volume of a contained gas will increase its. Does Increasing Pressure Increase Volume.
From www.slideserve.com
PPT The Haber Process PowerPoint Presentation, free download ID6194698 Does Increasing Pressure Increase Volume Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. This relationship, known as boyle's law, is one of the cornerstones of. Pressure is decreasing (from 2.44 atm. The relationship between pressure and volume: If you had a way to increase pressure with no volume change, then yes, temperature would increase. Does Increasing Pressure Increase Volume.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Does Increasing Pressure Increase Volume In fact, if the volume. We know that pressure and volume are inversely related; Pv=c p v = c. This relationship, known as boyle's law, is one of the cornerstones of. In reality, most compression take place by reducing volume or. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure.. Does Increasing Pressure Increase Volume.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Does Increasing Pressure Increase Volume This relationship, known as boyle's law, is one of the cornerstones of. As one decreases, the other increases. Pv=c p v = c. In fact, if the volume. In fact, if the volume. The relationship between pressure and volume: Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Pressure is. Does Increasing Pressure Increase Volume.
From www.slideserve.com
PPT Chemical Systems & Equilibrium PowerPoint Presentation, free Does Increasing Pressure Increase Volume Pressure is decreasing (from 2.44 atm. In fact, if the volume. In reality, most compression take place by reducing volume or. This relationship, known as boyle's law, is one of the cornerstones of. In fact, if the volume. Pv=c p v = c. The relationship between pressure and volume: Decreasing the volume of a contained gas will increase its pressure,. Does Increasing Pressure Increase Volume.
From www.proscubadiver.net
Gas Laws Formulas & Physics For Scuba Diving Does Increasing Pressure Increase Volume If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. If you lower the pressure, volume increases. In fact, if the volume. As one decreases, the other increases. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure.. Does Increasing Pressure Increase Volume.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing Does Increasing Pressure Increase Volume As the pressure on a gas increases, the volume of the gas decreases because the gas. This relationship, known as boyle's law, is one of the cornerstones of. The relationship between pressure and volume: In fact, if the volume. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. If you. Does Increasing Pressure Increase Volume.
From exonukltq.blob.core.windows.net
How Do You Increase Pressure On A Gas at Rebecca Craig blog Does Increasing Pressure Increase Volume If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. If you had a way to increase pressure with no volume change, then yes, temperature would increase by. Does Increasing Pressure Increase Volume.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Increasing Pressure Increase Volume If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. In reality, most compression take place by reducing volume or. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Pv=c p v = c.. Does Increasing Pressure Increase Volume.
From www.alamy.com
Pressure volume curve, increased intracranial pressure, csf, arterial Does Increasing Pressure Increase Volume Pv=c p v = c. We know that pressure and volume are inversely related; If you lower the pressure, volume increases. As the pressure on a gas increases, the volume of the gas decreases because the gas. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the. Does Increasing Pressure Increase Volume.
From chemistry.stackexchange.com
gas laws Pressure Vs Volume plot for real and ideal gasses Does Increasing Pressure Increase Volume In fact, if the volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. We know that pressure and volume are inversely related; Pressure is decreasing (from 2.44. Does Increasing Pressure Increase Volume.
From www.chegg.com
Solved Data and Calculations for Pressure Volume Does Increasing Pressure Increase Volume In fact, if the volume. As the pressure on a gas increases, the volume of the gas decreases because the gas. Where c is a constant. Pressure is decreasing (from 2.44 atm. If you lower the pressure, volume increases. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. As one. Does Increasing Pressure Increase Volume.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Increasing Pressure Increase Volume As the pressure on a gas increases, the volume of the gas decreases because the gas. As one decreases, the other increases. If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. In fact, if the volume. We know that pressure and volume are inversely related; In. Does Increasing Pressure Increase Volume.
From www.slideserve.com
PPT AP Chemistry Unit 2 PowerPoint Presentation, free download ID Does Increasing Pressure Increase Volume As the pressure on a gas increases, the volume of the gas decreases because the gas. In fact, if the volume. If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. If you lower the pressure, volume increases. Where c is a constant. Decreasing the volume of. Does Increasing Pressure Increase Volume.
From www.daxor.com
Why is Blood Volume Analysis Important? Daxor Does Increasing Pressure Increase Volume This relationship, known as boyle's law, is one of the cornerstones of. As the pressure on a gas increases, the volume of the gas decreases because the gas. In fact, if the volume. If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. If you had a. Does Increasing Pressure Increase Volume.
From www.slideserve.com
PPT Blood Pressure PowerPoint Presentation, free download ID3785699 Does Increasing Pressure Increase Volume Where c is a constant. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. We know that pressure and volume are inversely related; If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Pv=c p v = c.. Does Increasing Pressure Increase Volume.
From slidetodoc.com
The relationship between temperature and volume How Volume Does Increasing Pressure Increase Volume As one decreases, the other increases. Pressure is decreasing (from 2.44 atm. As the pressure on a gas increases, the volume of the gas decreases because the gas. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Pv=c p v = c. If you increase the pressure. Does Increasing Pressure Increase Volume.
From www.nagwa.com
Question Video Identifying the Relationship between the Pressure and Does Increasing Pressure Increase Volume In fact, if the volume. As one decreases, the other increases. If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be. Pressure is decreasing (from 2.44 atm. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. As. Does Increasing Pressure Increase Volume.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Does Increasing Pressure Increase Volume In fact, if the volume. In fact, if the volume. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In reality, most compression take place by reducing volume or. Pressure is decreasing (from 2.44 atm. If you lower the pressure, volume increases. The relationship between pressure and volume: Decreasing the. Does Increasing Pressure Increase Volume.
From quizlet.com
arterial blood volume and pressure Diagram Quizlet Does Increasing Pressure Increase Volume If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. As one decreases, the other increases. The relationship between pressure and volume: This relationship, known as boyle's law, is one of the cornerstones of. Where c is a constant. If you lower the pressure, volume increases. If you. Does Increasing Pressure Increase Volume.
From www.slideserve.com
PPT Chapter 19 Thermodynamics PowerPoint Presentation, free download Does Increasing Pressure Increase Volume In fact, if the volume. Pressure is decreasing (from 2.44 atm. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. This relationship, known as boyle's law, is one of the cornerstones of. The relationship between pressure and volume: In reality, most compression take place by reducing volume or. Where c. Does Increasing Pressure Increase Volume.
From www.adinstruments.com
PressureVolume Loop Relationships EDPVR and ESPVR ADInstruments Does Increasing Pressure Increase Volume Where c is a constant. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. We know that pressure and volume are inversely related; The relationship between pressure and volume: This relationship, known as boyle's law, is one of the cornerstones of. Pressure is decreasing (from 2.44 atm.. Does Increasing Pressure Increase Volume.
From www.slideserve.com
PPT Compressibility PowerPoint Presentation, free download ID4200029 Does Increasing Pressure Increase Volume The relationship between pressure and volume: If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. As the pressure on a gas increases, the volume of the gas decreases because the gas. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will. Does Increasing Pressure Increase Volume.