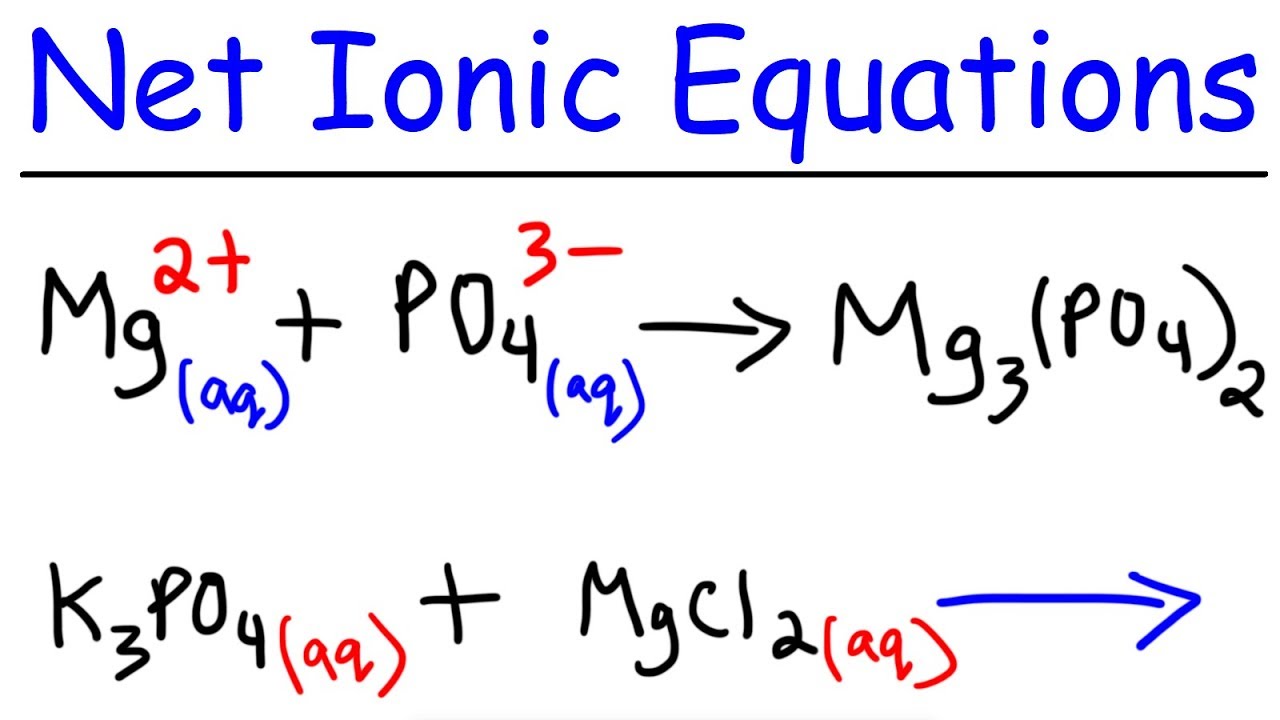Net Reaction Definition . Ions in a reaction that do not undergo any change. Ions (never words) will be used for the complete ionic equation and. The balanced equation will be. Enter an equation of an ionic chemical equation and press the balance button. A molecular equation that depicts the ions or molecules that experience a change within a reaction. In the net ionic equation, any. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Chemical reactions that occur in solution are most concisely described by writing net ionic equations. Only full formulas (never words or ions) are involved in a complete molecular equation. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. A solution in which the solvent is water. A net ionic equation is the most. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved.
from learningschoolphyllode.z5.web.core.windows.net
A net ionic equation is the most. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved. Chemical reactions that occur in solution are most concisely described by writing net ionic equations. A solution in which the solvent is water. In the net ionic equation, any. Enter an equation of an ionic chemical equation and press the balance button. Only full formulas (never words or ions) are involved in a complete molecular equation. Ions (never words) will be used for the complete ionic equation and.
Ionic And Net Ionic Equations Practice
Net Reaction Definition Ions (never words) will be used for the complete ionic equation and. Ions in a reaction that do not undergo any change. Only full formulas (never words or ions) are involved in a complete molecular equation. Ions (never words) will be used for the complete ionic equation and. Chemical reactions that occur in solution are most concisely described by writing net ionic equations. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved. The balanced equation will be. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. A net ionic equation is the most. In the net ionic equation, any. Enter an equation of an ionic chemical equation and press the balance button. A molecular equation that depicts the ions or molecules that experience a change within a reaction. A solution in which the solvent is water.
From www.researchgate.net
Mixing and netreaction plots. The product of the concentration and the Net Reaction Definition Ions in a reaction that do not undergo any change. In the net ionic equation, any. A net ionic equation is the most. Only full formulas (never words or ions) are involved in a complete molecular equation. Ions (never words) will be used for the complete ionic equation and. A molecular equation that depicts the ions or molecules that experience. Net Reaction Definition.
From www.researchgate.net
Reaction network diagram of the ErbB signaling model. Net reaction Net Reaction Definition A molecular equation that depicts the ions or molecules that experience a change within a reaction. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. In the net ionic equation, any. Chemical reactions that occur in solution are most concisely described by writing net ionic. Net Reaction Definition.
From www.coursehero.com
[Solved] determine the half reactions and net reaction and the minimum Net Reaction Definition Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved. A net ionic equation is the most. Ions (never words) will be used for the complete ionic equation and. A molecular equation that depicts the ions or molecules that experience a change within a reaction. In the. Net Reaction Definition.
From www.researchgate.net
Net reaction rate for mixture 3 at P1 = 20.0 torr and T5 = 1531 K. The Net Reaction Definition The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Ions in a reaction that do not undergo any change. A net ionic equation is the most. Enter an equation of an ionic chemical equation and press the balance button. A molecular equation that depicts the. Net Reaction Definition.
From aznswerzoneoxycopernican.z21.web.core.windows.net
Chemical Reaction In Acidbase Neutralization Net Reaction Definition Only full formulas (never words or ions) are involved in a complete molecular equation. Chemical reactions that occur in solution are most concisely described by writing net ionic equations. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. A solution in which the solvent is water. Ions (never words) will be used for. Net Reaction Definition.
From www.wikihow.com
How to Write a Net Ionic Equation 10 Steps (with Pictures) Net Reaction Definition The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Ions in a reaction that do not undergo any change. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. Enter an equation of an ionic chemical equation and press. Net Reaction Definition.
From dewwool.com
Neutralization ReactionDefinitionExamplesTypes DewWool Net Reaction Definition Only full formulas (never words or ions) are involved in a complete molecular equation. A molecular equation that depicts the ions or molecules that experience a change within a reaction. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. In the complete ionic equation, soluble. Net Reaction Definition.
From www.youtube.com
6.1.1 Define the term rate of reaction. YouTube Net Reaction Definition Ions (never words) will be used for the complete ionic equation and. A molecular equation that depicts the ions or molecules that experience a change within a reaction. Ions in a reaction that do not undergo any change. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates. Net Reaction Definition.
From www.youtube.com
ALEKS Writing the Net Equation for a Sequence of Reactions YouTube Net Reaction Definition A molecular equation that depicts the ions or molecules that experience a change within a reaction. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Only full formulas (never words or ions) are involved in a complete molecular equation. A net ionic equation is the. Net Reaction Definition.
From aznswerzonelisunanchored.z13.web.core.windows.net
How To Complete Neutralization Reactions Net Reaction Definition Only full formulas (never words or ions) are involved in a complete molecular equation. A molecular equation that depicts the ions or molecules that experience a change within a reaction. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. The balanced equation will be. Ions. Net Reaction Definition.
From www.teachoo.com
Neutralization Reaction Definition, Equation and Examples Teachoo Net Reaction Definition A net ionic equation is the most. Ions (never words) will be used for the complete ionic equation and. Enter an equation of an ionic chemical equation and press the balance button. In the net ionic equation, any. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. Only full formulas (never words or. Net Reaction Definition.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Net Reaction Definition Ions in a reaction that do not undergo any change. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved. Enter an equation of an ionic chemical equation and press the balance button. In the net ionic equation, any. In the complete ionic equation, soluble ionic compounds. Net Reaction Definition.
From www.coursehero.com
[Solved] PHOTOSYNTHESIS NET REACTION oxidation reduction Course Hero Net Reaction Definition Chemical reactions that occur in solution are most concisely described by writing net ionic equations. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. Enter an equation of an ionic chemical equation and press the balance button. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for. Net Reaction Definition.
From www.numerade.com
SOLVED The following chemical reaction takes place in aqueous solution Net Reaction Definition The balanced equation will be. Ions (never words) will be used for the complete ionic equation and. A net ionic equation is the most. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Chemical reactions that occur in solution are most concisely described by writing. Net Reaction Definition.
From www.chegg.com
Solved The net reaction of glycolysis is Glucose Net Reaction Definition The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Ions (never words) will be used for the complete ionic equation and. A net ionic equation is the most. Ions in a reaction that do not undergo any change. Chemical reactions that occur in solution are. Net Reaction Definition.
From learningschoolphyllode.z5.web.core.windows.net
Ionic And Net Ionic Equations Practice Net Reaction Definition Ions in a reaction that do not undergo any change. Only full formulas (never words or ions) are involved in a complete molecular equation. In the net ionic equation, any. A solution in which the solvent is water. Enter an equation of an ionic chemical equation and press the balance button. A net ionic equation is the most. A molecular. Net Reaction Definition.
From aznswerzonelisunanchored.z13.web.core.windows.net
How To Do Neutralization Reaction Net Reaction Definition A molecular equation that depicts the ions or molecules that experience a change within a reaction. In the net ionic equation, any. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Ions in a reaction that do not undergo any change. Net reactions refer to. Net Reaction Definition.
From saylordotorg.github.io
Precipitation Reactions Net Reaction Definition A molecular equation that depicts the ions or molecules that experience a change within a reaction. A net ionic equation is the most. Chemical reactions that occur in solution are most concisely described by writing net ionic equations. In the net ionic equation, any. Ions (never words) will be used for the complete ionic equation and. Enter an equation of. Net Reaction Definition.
From www.researchgate.net
Schematic representation of the interplay between the net reaction flux Net Reaction Definition The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Ions (never words) will be used for the complete ionic equation and. Enter an equation of an ionic chemical equation and press the balance button. A solution in which the solvent is water. Chemical reactions that. Net Reaction Definition.
From www.slideserve.com
PPT Carbohydrate Metabolism in Plants PowerPoint Presentation, free Net Reaction Definition Enter an equation of an ionic chemical equation and press the balance button. A molecular equation that depicts the ions or molecules that experience a change within a reaction. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved. The net ionic equation is the chemical equation. Net Reaction Definition.
From chem.libretexts.org
13.4 Conjugated πsystems Chemistry LibreTexts Net Reaction Definition A molecular equation that depicts the ions or molecules that experience a change within a reaction. A net ionic equation is the most. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved. Chemical reactions that occur in solution are most concisely described by writing net ionic. Net Reaction Definition.
From www.researchgate.net
(a) modeling net reaction rates for the main individual Net Reaction Definition A net ionic equation is the most. The balanced equation will be. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Only full formulas (never words or ions) are involved in a complete molecular equation. Chemical reactions that occur in solution are most concisely described. Net Reaction Definition.
From www.researchgate.net
(a) net reaction of benzene and Cl 2 ; (b) the currently accepted Net Reaction Definition A molecular equation that depicts the ions or molecules that experience a change within a reaction. Ions (never words) will be used for the complete ionic equation and. Only full formulas (never words or ions) are involved in a complete molecular equation. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. Chemical reactions. Net Reaction Definition.
From www.researchgate.net
Net reaction rates comparisons of NO formation reaction in the thermal Net Reaction Definition Ions in a reaction that do not undergo any change. In the net ionic equation, any. The balanced equation will be. Enter an equation of an ionic chemical equation and press the balance button. Ions (never words) will be used for the complete ionic equation and. Chemical reactions that occur in solution are most concisely described by writing net ionic. Net Reaction Definition.
From www.expii.com
Reaction Mechanism — Definition & Elementary Steps Expii Net Reaction Definition The balanced equation will be. Ions (never words) will be used for the complete ionic equation and. Chemical reactions that occur in solution are most concisely described by writing net ionic equations. In the net ionic equation, any. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the. Net Reaction Definition.
From www.youtube.com
salt hydrolysis and net ionic equations review YouTube Net Reaction Definition In the net ionic equation, any. Ions in a reaction that do not undergo any change. A solution in which the solvent is water. In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. Enter an equation of an ionic chemical equation and press the balance button. Only full formulas (never words or ions). Net Reaction Definition.
From chem.libretexts.org
13.4 Conjugated πsystems Chemistry LibreTexts Net Reaction Definition Ions in a reaction that do not undergo any change. Chemical reactions that occur in solution are most concisely described by writing net ionic equations. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved. The balanced equation will be. Ions (never words) will be used for. Net Reaction Definition.
From www.tessshebaylo.com
Ammonium Iodide Dissolved In Water Net Ionic Equation Tessshebaylo Net Reaction Definition Enter an equation of an ionic chemical equation and press the balance button. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. Only full formulas (never words or ions) are involved in a complete molecular equation. In the net ionic equation, any. A net ionic. Net Reaction Definition.
From www.researchgate.net
Phthalide net reaction rate at the smface, and bulk conditions and Net Reaction Definition Ions in a reaction that do not undergo any change. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved. In the net ionic equation, any. The balanced equation will be. Ions (never words) will be used for the complete ionic equation and. Only full formulas (never. Net Reaction Definition.
From www.researchgate.net
Net reaction rates and liquid composition profiles for Example I (a Net Reaction Definition In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. Ions (never words) will be used for the complete ionic equation and. Chemical reactions that occur in solution are most concisely described by writing net ionic equations. Only full formulas (never words or ions) are involved in a complete molecular equation. A solution in. Net Reaction Definition.
From www.slideserve.com
PPT Net Ionic Equation PowerPoint Presentation, free download ID Net Reaction Definition In the complete ionic equation, soluble ionic compounds and strong acids are rewritten as dissociated ions. Enter an equation of an ionic chemical equation and press the balance button. A net ionic equation is the most. Net reactions refer to the overall chemical change that occurs in a reaction after accounting for all the individual steps or intermediates involved. A. Net Reaction Definition.
From sciencenotes.org
Neutralization Reaction Definition and Products Net Reaction Definition Enter an equation of an ionic chemical equation and press the balance button. A molecular equation that depicts the ions or molecules that experience a change within a reaction. Ions in a reaction that do not undergo any change. A net ionic equation is the most. Net reactions refer to the overall chemical change that occurs in a reaction after. Net Reaction Definition.
From www.researchgate.net
Schematic diagram showing net reaction rates for three elementary steps Net Reaction Definition Ions in a reaction that do not undergo any change. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction. In the net ionic equation, any. Ions (never words) will be used for the complete ionic equation and. A net ionic equation is the most. Chemical. Net Reaction Definition.
From narodnatribuna.info
Neutralization Reaction Definition Equation Examples Net Reaction Definition Only full formulas (never words or ions) are involved in a complete molecular equation. A molecular equation that depicts the ions or molecules that experience a change within a reaction. A net ionic equation is the most. Enter an equation of an ionic chemical equation and press the balance button. Chemical reactions that occur in solution are most concisely described. Net Reaction Definition.
From www.chegg.com
Solved Consider the following overall (or net) reaction Net Reaction Definition In the net ionic equation, any. A molecular equation that depicts the ions or molecules that experience a change within a reaction. Ions in a reaction that do not undergo any change. The balanced equation will be. Only full formulas (never words or ions) are involved in a complete molecular equation. In the complete ionic equation, soluble ionic compounds and. Net Reaction Definition.