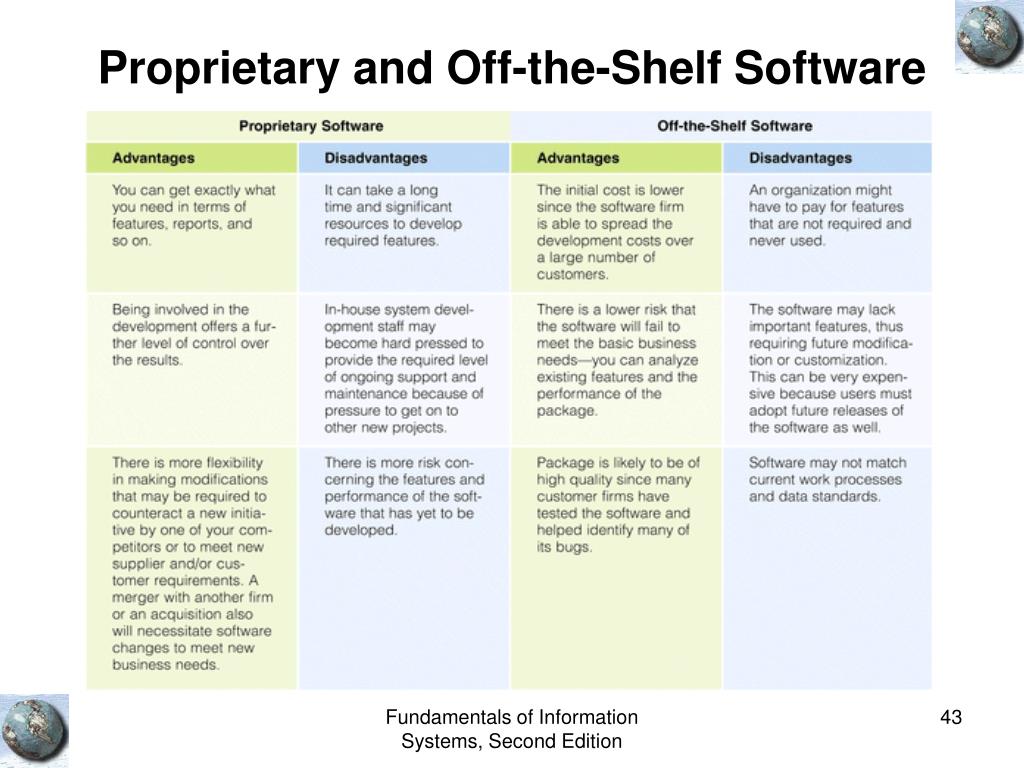
Off The Shelf Software Guidance Fda . Guidance for the content of premarket submissions for software. This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Center for devices and radiological health.
from www.slideserve.com
This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Center for devices and radiological health. Guidance for the content of premarket submissions for software.
PPT Hardware and Software PowerPoint Presentation, free download ID
Off The Shelf Software Guidance Fda Relevant fda guidance and/or supportive publications*. Relevant fda guidance and/or supportive publications*. This guidance document provides information regarding the recommended documentation sponsors should include in a. Guidance for the content of premarket submissions for software. Center for devices and radiological health. A growing number of medical devices are designed to be connected.
From www.mavensolutions.tech
How does offtheshelf software hinder growth? Maven Solutions Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. Center for devices and radiological health. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. Off The Shelf Software Guidance Fda.
From dignitas.digital
Using off the shelf solution or Custom Software Dignitas Digital Off The Shelf Software Guidance Fda Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Center for devices and radiological health. Guidance for the content of premarket submissions for software. This guidance document provides information regarding the recommended documentation sponsors should include in a. Off The Shelf Software Guidance Fda.
From itenterprise.co.uk
The Complete Advantages and Disadvantages of Off the Shelf Software Off The Shelf Software Guidance Fda Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. This guidance document provides information regarding the recommended documentation sponsors should include in a. Center for devices and radiological health. Guidance for the content of premarket submissions for software. Off The Shelf Software Guidance Fda.
From simpat.tech
5 OffTheShelf Software Advantages and Disadvantages Simpat Tech Off The Shelf Software Guidance Fda Guidance for the content of premarket submissions for software. Center for devices and radiological health. A growing number of medical devices are designed to be connected. This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. Off The Shelf Software Guidance Fda.
From issuu.com
Difference Between Proprietary Software and OffTheShelf Software by Off The Shelf Software Guidance Fda Center for devices and radiological health. Guidance for the content of premarket submissions for software. This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Off The Shelf Software Guidance Fda.
From www.regdesk.co
FDA Revised Guidance on OffTheShelf Software Use Maintenance and Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. Guidance for the content of premarket submissions for software. A growing number of medical devices are designed to be connected. Center for devices and radiological health. Off The Shelf Software Guidance Fda.
From itechnolabs.ca
What is a COTS Software [Updated 2024] Off The Shelf Software Guidance Fda Relevant fda guidance and/or supportive publications*. Center for devices and radiological health. Guidance for the content of premarket submissions for software. A growing number of medical devices are designed to be connected. This guidance document provides information regarding the recommended documentation sponsors should include in a. Off The Shelf Software Guidance Fda.
From sparkbusinessworks.com
Building Custom Software vs OfftheShelf Advantages and Disadvantages Off The Shelf Software Guidance Fda Guidance for the content of premarket submissions for software. This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Center for devices and radiological health. Off The Shelf Software Guidance Fda.
From www.complianceonline.com
Guidance for OfftheShelf Software Use in Medical Devices Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. Center for devices and radiological health. Relevant fda guidance and/or supportive publications*. Off The Shelf Software Guidance Fda.
From www.regdesk.co
FDA Guidance on OffTheShelf Software Use in Medical Devices RegDesk Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. Center for devices and radiological health. Off The Shelf Software Guidance Fda.
From joifotpvz.blob.core.windows.net
Commercial Off The Shelf Software S Are Also Called at David Matthews blog Off The Shelf Software Guidance Fda A growing number of medical devices are designed to be connected. This guidance document provides information regarding the recommended documentation sponsors should include in a. Center for devices and radiological health. Relevant fda guidance and/or supportive publications*. Guidance for the content of premarket submissions for software. Off The Shelf Software Guidance Fda.
From invozone.com
Why Should and Shouldn’t I Use Off the Shelf Software? Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. Guidance for the content of premarket submissions for software. Center for devices and radiological health. A growing number of medical devices are designed to be connected. Off The Shelf Software Guidance Fda.
From exoungkyt.blob.core.windows.net
OffTheShelf Software Solutions at Rose Benson blog Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. A growing number of medical devices are designed to be connected. Relevant fda guidance and/or supportive publications*. Center for devices and radiological health. Guidance for the content of premarket submissions for software. Off The Shelf Software Guidance Fda.
From andersenlab.com
Custom Software vs OfftheShelf Pros & Cons Off The Shelf Software Guidance Fda Guidance for the content of premarket submissions for software. Center for devices and radiological health. This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Off The Shelf Software Guidance Fda.
From www.regdesk.co
FDA Revised Guidance on OffTheShelf Software Risk Assessment and Off The Shelf Software Guidance Fda Center for devices and radiological health. A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. Off The Shelf Software Guidance Fda.
From innolitics.com
OffTheShelf Software Best Practices, FAQs, and Examples Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. Guidance for the content of premarket submissions for software. A growing number of medical devices are designed to be connected. Relevant fda guidance and/or supportive publications*. Center for devices and radiological health. Off The Shelf Software Guidance Fda.
From diceus.com
Custom vs OfftheShelf Software Advantages & Disadvantages Off The Shelf Software Guidance Fda Relevant fda guidance and/or supportive publications*. This guidance document provides information regarding the recommended documentation sponsors should include in a. A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. Center for devices and radiological health. Off The Shelf Software Guidance Fda.
From www.regdesk.co
FDA Guidance on OffTheShelf Software Use in Medical Devices RegDesk Off The Shelf Software Guidance Fda Relevant fda guidance and/or supportive publications*. This guidance document provides information regarding the recommended documentation sponsors should include in a. A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. Center for devices and radiological health. Off The Shelf Software Guidance Fda.
From www.regdesk.co
FDA Revised Guidance on OffTheShelf Software Use Introduction RegDesk Off The Shelf Software Guidance Fda Center for devices and radiological health. Guidance for the content of premarket submissions for software. This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Off The Shelf Software Guidance Fda.
From www.thirdrocktechkno.com
Custom vs Offtheshelf software solution A Comparative Guide Off The Shelf Software Guidance Fda Guidance for the content of premarket submissions for software. Relevant fda guidance and/or supportive publications*. Center for devices and radiological health. This guidance document provides information regarding the recommended documentation sponsors should include in a. A growing number of medical devices are designed to be connected. Off The Shelf Software Guidance Fda.
From www.koder.ly
Offtheshelf Software vs Bespoke Software Off The Shelf Software Guidance Fda Guidance for the content of premarket submissions for software. Relevant fda guidance and/or supportive publications*. Center for devices and radiological health. A growing number of medical devices are designed to be connected. This guidance document provides information regarding the recommended documentation sponsors should include in a. Off The Shelf Software Guidance Fda.
From www.scribd.com
FDA Guidance OffTheShelf PDF Operating System Medical Device Off The Shelf Software Guidance Fda Guidance for the content of premarket submissions for software. Center for devices and radiological health. A growing number of medical devices are designed to be connected. Relevant fda guidance and/or supportive publications*. This guidance document provides information regarding the recommended documentation sponsors should include in a. Off The Shelf Software Guidance Fda.
From xbsoftware.com
Custom Software vs OfftheShelf Software XB Software Off The Shelf Software Guidance Fda Guidance for the content of premarket submissions for software. Center for devices and radiological health. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. This guidance document provides information regarding the recommended documentation sponsors should include in a. Off The Shelf Software Guidance Fda.
From morioh.com
Custom Software vs Offtheshelf Software How to select a better one Off The Shelf Software Guidance Fda Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Center for devices and radiological health. Guidance for the content of premarket submissions for software. This guidance document provides information regarding the recommended documentation sponsors should include in a. Off The Shelf Software Guidance Fda.
From innolitics.com
2019 FDA Guidance OffTheShelf Software Use in Medical Devices Off The Shelf Software Guidance Fda A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. Center for devices and radiological health. Relevant fda guidance and/or supportive publications*. This guidance document provides information regarding the recommended documentation sponsors should include in a. Off The Shelf Software Guidance Fda.
From medium.com
Custom Software vs OfftheShelf Software Which One to Choose? by Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. A growing number of medical devices are designed to be connected. Center for devices and radiological health. Guidance for the content of premarket submissions for software. Relevant fda guidance and/or supportive publications*. Off The Shelf Software Guidance Fda.
From www.slideserve.com
PPT Hardware and Software PowerPoint Presentation, free download ID Off The Shelf Software Guidance Fda A growing number of medical devices are designed to be connected. Relevant fda guidance and/or supportive publications*. This guidance document provides information regarding the recommended documentation sponsors should include in a. Center for devices and radiological health. Guidance for the content of premarket submissions for software. Off The Shelf Software Guidance Fda.
From issuu.com
Guidance for off the shelf software use in medical device by Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. Guidance for the content of premarket submissions for software. Relevant fda guidance and/or supportive publications*. Center for devices and radiological health. A growing number of medical devices are designed to be connected. Off The Shelf Software Guidance Fda.
From innolitics.com
2019 FDA Guidance OffTheShelf Software Use in Medical Devices Off The Shelf Software Guidance Fda Center for devices and radiological health. A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. Off The Shelf Software Guidance Fda.
From exoungkyt.blob.core.windows.net
OffTheShelf Software Solutions at Rose Benson blog Off The Shelf Software Guidance Fda Center for devices and radiological health. A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. Relevant fda guidance and/or supportive publications*. This guidance document provides information regarding the recommended documentation sponsors should include in a. Off The Shelf Software Guidance Fda.
From moreapp.com
OfftheShelf Software vs Custom Software MoreApp Blog Off The Shelf Software Guidance Fda Guidance for the content of premarket submissions for software. Center for devices and radiological health. A growing number of medical devices are designed to be connected. This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. Off The Shelf Software Guidance Fda.
From diceus.com
Off the shelf software and Custom Software what Is the Main Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Center for devices and radiological health. Guidance for the content of premarket submissions for software. Off The Shelf Software Guidance Fda.
From www.botreetechnologies.com
Customized Software What is it, Types, and Examples Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. A growing number of medical devices are designed to be connected. Guidance for the content of premarket submissions for software. Relevant fda guidance and/or supportive publications*. Center for devices and radiological health. Off The Shelf Software Guidance Fda.
From congenius.ch
OffTheShelf software in medical devices FDA guidance Congenius Off The Shelf Software Guidance Fda This guidance document provides information regarding the recommended documentation sponsors should include in a. Guidance for the content of premarket submissions for software. Relevant fda guidance and/or supportive publications*. A growing number of medical devices are designed to be connected. Center for devices and radiological health. Off The Shelf Software Guidance Fda.
From www.web-alliance.co.uk
Pros and cons of offtheshelf software. Off The Shelf Software Guidance Fda A growing number of medical devices are designed to be connected. This guidance document provides information regarding the recommended documentation sponsors should include in a. Guidance for the content of premarket submissions for software. Relevant fda guidance and/or supportive publications*. Center for devices and radiological health. Off The Shelf Software Guidance Fda.