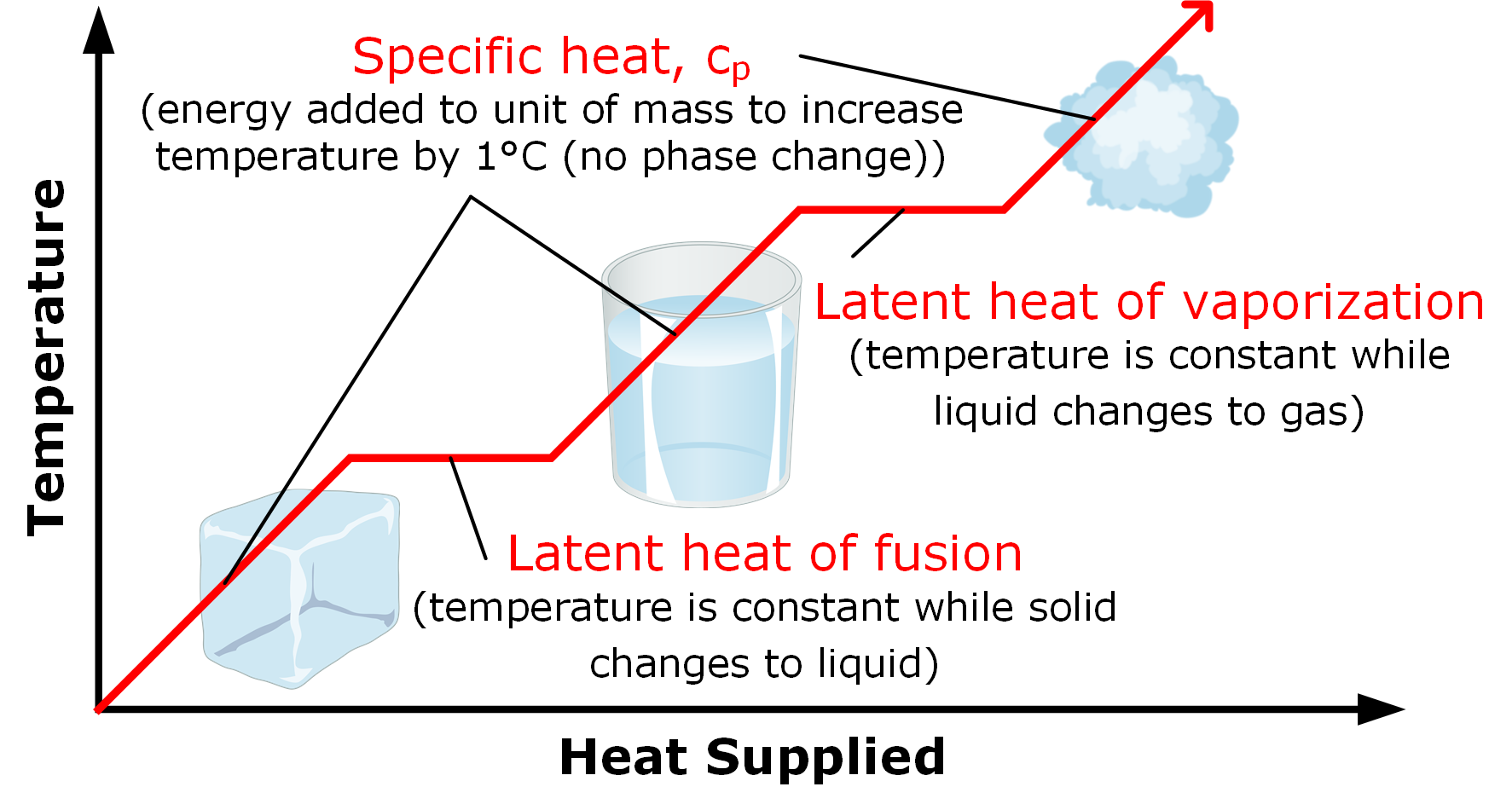
Solid To Liquid Heat Of Fusion . The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. When studying chemistry, “fusion” simply has the same definition as melting. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. Since the melting of any. It's also known as enthalpy of fusion.
from guides.co
Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. It's also known as enthalpy of fusion. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. When studying chemistry, “fusion” simply has the same definition as melting. Since the melting of any.
Phase Change Requires Heat FD2021 Fundamentals of Fire and
Solid To Liquid Heat Of Fusion Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. When studying chemistry, “fusion” simply has the same definition as melting. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. It's also known as enthalpy of fusion. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. Since the melting of any.
From www.slideserve.com
PPT The Nature of Water PowerPoint Presentation, free download ID Solid To Liquid Heat Of Fusion Since the melting of any. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The heat which a solid absorbs when. Solid To Liquid Heat Of Fusion.
From www.linstitute.net
AQA A Level Physics复习笔记6.4.4 Latent Heat Capacity翰林国际教育 Solid To Liquid Heat Of Fusion Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. When studying chemistry, “fusion” simply has the same. Solid To Liquid Heat Of Fusion.
From www.slideserve.com
PPT CHAPTER 1 MATTER IN OUR SURROUNDINGS PowerPoint Presentation Solid To Liquid Heat Of Fusion Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a. Solid To Liquid Heat Of Fusion.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2841621 Solid To Liquid Heat Of Fusion The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. It's also known as enthalpy of fusion. Since the melting of any. When studying chemistry, “fusion” simply has the same definition as melting. The concept of heat of fusion, often denoted by the. Solid To Liquid Heat Of Fusion.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid To Liquid Heat Of Fusion The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. It's also known as enthalpy of fusion. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Since the melting of any. Solids can. Solid To Liquid Heat Of Fusion.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Heat Of Fusion It's also known as enthalpy of fusion. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. When studying chemistry, “fusion” simply has the same definition as melting. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted. Solid To Liquid Heat Of Fusion.
From www.ck12.org
Heat of fusion Overview ( Video ) Chemistry CK12 Foundation Solid To Liquid Heat Of Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. When studying chemistry, “fusion” simply has the same definition as melting. Solids can be heated to the point where the molecules holding their bonds together break apart and form a. Solid To Liquid Heat Of Fusion.
From guides.co
Phase Change Requires Heat FD2021 Fundamentals of Fire and Solid To Liquid Heat Of Fusion It's also known as enthalpy of fusion. When studying chemistry, “fusion” simply has the same definition as melting. Since the melting of any. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. Heat of fusion, also called enthalpy of fusion or latent heat of. Solid To Liquid Heat Of Fusion.
From www.youtube.com
Physics Energy Heat Transfer Solids Liquids and Gases YouTube Solid To Liquid Heat Of Fusion The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. When. Solid To Liquid Heat Of Fusion.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet Solid To Liquid Heat Of Fusion Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The concept of heat of fusion, often denoted. Solid To Liquid Heat Of Fusion.
From ch301.cm.utexas.edu
heating curve Solid To Liquid Heat Of Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. When studying chemistry, “fusion” simply has the same definition as melting. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. It's also known. Solid To Liquid Heat Of Fusion.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid To Liquid Heat Of Fusion It's also known as enthalpy of fusion. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is. Solid To Liquid Heat Of Fusion.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Solid To Liquid Heat Of Fusion The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. Since the melting of any. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the. Solid To Liquid Heat Of Fusion.
From gamesmartz.com
Heat Of Fusion Definition & Image GameSmartz Solid To Liquid Heat Of Fusion Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat. Solid To Liquid Heat Of Fusion.
From www.slideserve.com
PPT Physical Behavior of Matter PowerPoint Presentation, free Solid To Liquid Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. Since the melting of any. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion. Solid To Liquid Heat Of Fusion.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Solid To Liquid Heat Of Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of. Solid To Liquid Heat Of Fusion.
From www.ck12.org
Heat of fusion Example 1 ( Video ) Chemistry CK12 Foundation Solid To Liquid Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. Since. Solid To Liquid Heat Of Fusion.
From streetlink.org.uk
💐 How to find heat of fusion. Heat of Fusion Explained. 20221224 Solid To Liquid Heat Of Fusion The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Solids can be. Solid To Liquid Heat Of Fusion.
From apollo.lsc.vsc.edu
Latent Heat of evaporation, fusion, and freezing Solid To Liquid Heat Of Fusion The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. Since the melting of any. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. Heat of fusion is the amount of. Solid To Liquid Heat Of Fusion.
From www.meritnation.com
The latent heat of fusion of ice is 5 99kjmol at its melting point Then Solid To Liquid Heat Of Fusion The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on. Solid To Liquid Heat Of Fusion.
From www.thoughtco.com
Heat of Fusion Example Problem Melting Ice Solid To Liquid Heat Of Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Since the melting of any. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a. Solid To Liquid Heat Of Fusion.
From stock.adobe.com
Thermal expansion of solids and liquids. The tendency of materials to Solid To Liquid Heat Of Fusion Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. It's also known as enthalpy of fusion. Since the melting of any. The concept of. Solid To Liquid Heat Of Fusion.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid To Liquid Heat Of Fusion The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. Since the melting of any. The concept of. Solid To Liquid Heat Of Fusion.
From www.slideserve.com
PPT Ch. 3.1 Solids, Liquids, Gases PowerPoint Presentation, free Solid To Liquid Heat Of Fusion The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. When studying chemistry, “fusion” simply has the same definition as melting. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance. Solid To Liquid Heat Of Fusion.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Solid To Liquid Heat Of Fusion The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. When studying chemistry, “fusion” simply has the same definition as melting. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. It's. Solid To Liquid Heat Of Fusion.
From www.slideserve.com
PPT To cause the state of matter to change…. PowerPoint Presentation Solid To Liquid Heat Of Fusion The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of. Solid To Liquid Heat Of Fusion.
From www.vedantu.com
Which Liquid Solid on Heating Learn Important Terms and Concepts Solid To Liquid Heat Of Fusion The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. Since the melting of any. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of. Solid To Liquid Heat Of Fusion.
From www.teachoo.com
Latent Heat of Vaporization and Fusion Definition Teachoo Solid To Liquid Heat Of Fusion Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. The molar heat of fusion. Solid To Liquid Heat Of Fusion.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Solid To Liquid Heat Of Fusion The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. The concept of. Solid To Liquid Heat Of Fusion.
From www.britannica.com
Latent heat Definition, Examples, & Facts Britannica Solid To Liquid Heat Of Fusion The concept of heat of fusion, often denoted by the symbol δh fus, is a critical. It's also known as enthalpy of fusion. Since the melting of any. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquid. The molar heat of fusion \(\left( \delta. Solid To Liquid Heat Of Fusion.
From www.pinterest.com
INFO SHEET Hidden heat Converting a liquid at its boiling point to Solid To Liquid Heat Of Fusion The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. It's also known as enthalpy of fusion. When studying chemistry, “fusion” simply has. Solid To Liquid Heat Of Fusion.
From childhealthpolicy.vumc.org
🏆 How to find heat of fusion. Heat of Sublimation. 20221010 Solid To Liquid Heat Of Fusion The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance is the heat absorbed by one mole of that substance as it is converted from a solid to a liquid. Since the melting of any. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted. Solid To Liquid Heat Of Fusion.
From www.chegg.com
Solved Substance X is known to exist at 1 atm in the solid, Solid To Liquid Heat Of Fusion It's also known as enthalpy of fusion. When studying chemistry, “fusion” simply has the same definition as melting. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. The molar heat of fusion \(\left( \delta h_\text{fus} \right)\) of a substance. Solid To Liquid Heat Of Fusion.
From study.com
Heat of Fusion Definition, Equation & Examples Video & Lesson Solid To Liquid Heat Of Fusion When studying chemistry, “fusion” simply has the same definition as melting. Solids can be heated to the point where the molecules holding their bonds together break apart and form a liquid. It's also known as enthalpy of fusion. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or. Solid To Liquid Heat Of Fusion.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation, free download ID2249917 Solid To Liquid Heat Of Fusion Since the melting of any. When studying chemistry, “fusion” simply has the same definition as melting. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar basis. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of. Solid To Liquid Heat Of Fusion.