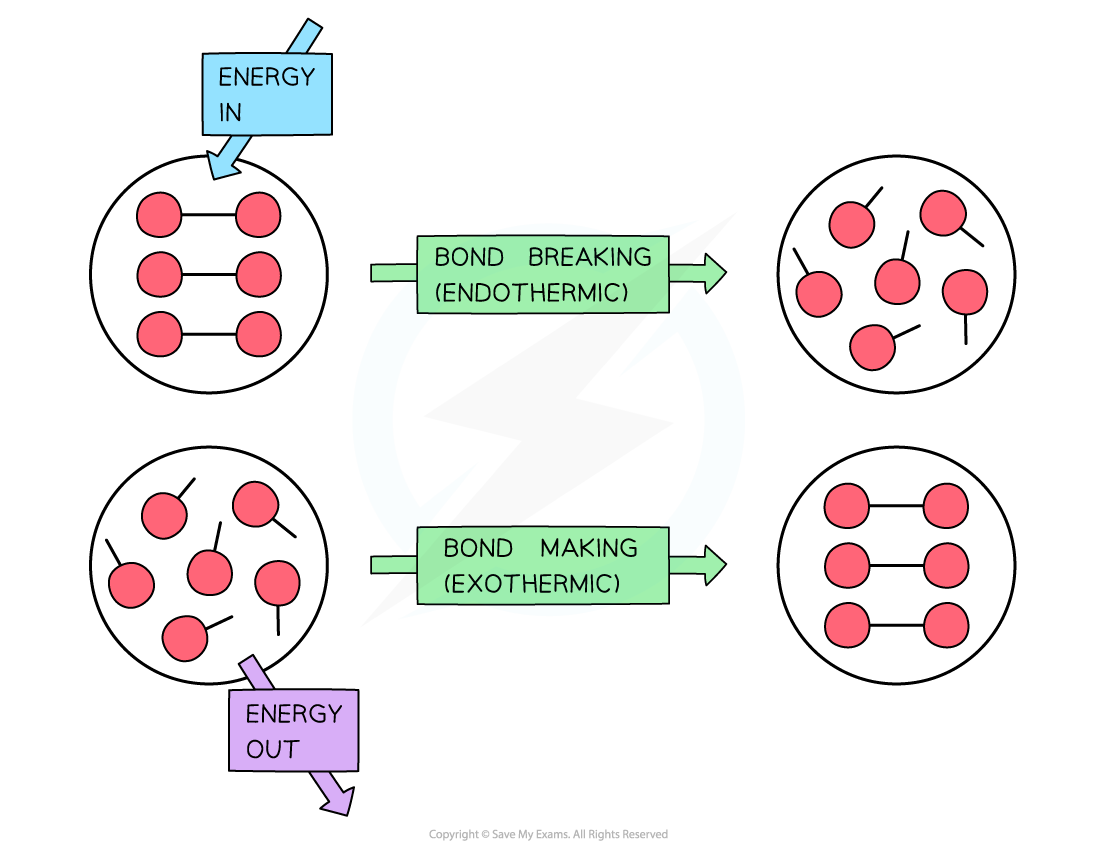
Why Are Double Bonds Easier To Break . Yes, we're breaking the double bond in cyclohexene. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. In this section, you will learn. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Look at these three examples of branched hexane derivatives (with. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also. But it gets more complicated. Branching decreases melting point and boiling point. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond.
from www.linstitute.net
But it gets more complicated. Look at these three examples of branched hexane derivatives (with. Branching decreases melting point and boiling point. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also. In this section, you will learn. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. Yes, we're breaking the double bond in cyclohexene.
CIE A Level Chemistry复习笔记1.5.4 Enthalpy & Bond Energies翰林国际教育
Why Are Double Bonds Easier To Break In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Look at these three examples of branched hexane derivatives (with. In this section, you will learn. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. Yes, we're breaking the double bond in cyclohexene. Branching decreases melting point and boiling point. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. But it gets more complicated. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds.
From ar.inspiredpencil.com
Examples Of Double Bonds In Chemistry Why Are Double Bonds Easier To Break In this section, you will learn. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. Yes, we're breaking the double bond in cyclohexene. Branching decreases melting point and boiling point. But it gets more complicated. The shorter bond length in double bonds results. Why Are Double Bonds Easier To Break.
From www.youtube.com
Double Bonds Do Not Rotate YouTube Why Are Double Bonds Easier To Break Look at these three examples of branched hexane derivatives (with. But it gets more complicated. Branching decreases melting point and boiling point. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also. In this section, you will learn.. Why Are Double Bonds Easier To Break.
From asiasupergrid.com
What Is A Double Bond Example Why Are Double Bonds Easier To Break A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. But it gets more complicated. Look at these three examples. Why Are Double Bonds Easier To Break.
From www.youtube.com
double bond equivalent degree of carbon class12&11 shortvedio Why Are Double Bonds Easier To Break The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. In this section, you will learn. Branching decreases melting point. Why Are Double Bonds Easier To Break.
From asiasupergrid.com
What Is A Double Bond Example Why Are Double Bonds Easier To Break Yes, we're breaking the double bond in cyclohexene. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Look at these three examples of branched hexane derivatives (with. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely. Why Are Double Bonds Easier To Break.
From ask.modifiyegaraj.com
Double And Triple Bonds Form Because Asking List Why Are Double Bonds Easier To Break But it gets more complicated. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. Look at these three examples of branched hexane derivatives (with. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. Why Are Double Bonds Easier To Break.
From www.vrogue.co
Naming Organic Compounds With Double And Triple Bonds vrogue.co Why Are Double Bonds Easier To Break Branching decreases melting point and boiling point. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. Yes, we're breaking the double bond in cyclohexene. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single. Why Are Double Bonds Easier To Break.
From www.youtube.com
Double and triple covalent bonds YouTube Why Are Double Bonds Easier To Break But it gets more complicated. In this section, you will learn. Yes, we're breaking the double bond in cyclohexene. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also. Look at these three examples of branched hexane derivatives. Why Are Double Bonds Easier To Break.
From www.youtube.com
A.8.4 Describe conjugation of double bonds and wavelength of absorption Why Are Double Bonds Easier To Break In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. In this section, you will learn. Energy required to break double bond is 614. Why Are Double Bonds Easier To Break.
From byjus.com
Does CO2 have double bond Why Are Double Bonds Easier To Break A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Yes, we're breaking the double bond in cyclohexene. Branching decreases melting point and boiling point. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to. Why Are Double Bonds Easier To Break.
From www.showme.com
Double Bonds Science, Chemistry, Chemical Bonds ShowMe Why Are Double Bonds Easier To Break In this section, you will learn. But it gets more complicated. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. Look at these three examples of branched hexane derivatives (with. Branching decreases melting point and boiling point. In chemistry, a double bond is a covalent bond between. Why Are Double Bonds Easier To Break.
From ar.inspiredpencil.com
Bond Length Why Are Double Bonds Easier To Break In this section, you will learn. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms.. Why Are Double Bonds Easier To Break.
From definitionklw.blogspot.com
Double Bond Definition Chemistry DEFINITION KLW Why Are Double Bonds Easier To Break Branching decreases melting point and boiling point. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also. Look at these three examples of branched hexane derivatives (with. In chemistry, a double bond is a covalent bond between two. Why Are Double Bonds Easier To Break.
From www.animalia-life.club
Double Bond Why Are Double Bonds Easier To Break In this section, you will learn. Branching decreases melting point and boiling point. But it gets more complicated. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Energy required to break double bond is 614 j while in breaking single bond. Why Are Double Bonds Easier To Break.
From www.slideserve.com
PPT A Gentle Introduction to (or review of) Fundamentals of Chemistry Why Are Double Bonds Easier To Break Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also. In chemistry,. Why Are Double Bonds Easier To Break.
From www.youtube.com
Degree of unsaturation(Du)Or, Double bond, Equivalent(D.B.E), Hydrogen Why Are Double Bonds Easier To Break In this section, you will learn. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Branching decreases melting point. Why Are Double Bonds Easier To Break.
From www.shutterstock.com
3 Conjugated Double Bonds RoyaltyFree Photos and Stock Images Why Are Double Bonds Easier To Break But it gets more complicated. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. In this section,. Why Are Double Bonds Easier To Break.
From www.researchgate.net
Double bonds with multiwirebonds for the return path. Download Why Are Double Bonds Easier To Break But it gets more complicated. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double. Why Are Double Bonds Easier To Break.
From bio1151b.nicerweb.net
fatunsaturated.html 05_12bSaturatedUnsatFatL.jpg Why Are Double Bonds Easier To Break Look at these three examples of branched hexane derivatives (with. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to. Why Are Double Bonds Easier To Break.
From pediaa.com
Difference Between Single Double and Triple Bonds Definition Why Are Double Bonds Easier To Break But it gets more complicated. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. In chemistry, a double bond. Why Are Double Bonds Easier To Break.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.5.4 Enthalpy & Bond Energies翰林国际教育 Why Are Double Bonds Easier To Break Branching decreases melting point and boiling point. Look at these three examples of branched hexane derivatives (with. Yes, we're breaking the double bond in cyclohexene. But it gets more complicated. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. Triple bonds between like atoms are shorter than. Why Are Double Bonds Easier To Break.
From slideplayer.com
Covalent Bonding Review ppt download Why Are Double Bonds Easier To Break In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. Yes, we're breaking the double bond in cyclohexene. Branching decreases melting point and boiling. Why Are Double Bonds Easier To Break.
From slideplayer.com
Covalent Bonding. ppt download Why Are Double Bonds Easier To Break In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Yes, we're breaking the double bond in cyclohexene. Branching decreases melting point and boiling point. In this section, you will learn. A bond’s strength describes how strongly each atom is joined to another atom, and therefore. Why Are Double Bonds Easier To Break.
From www.thoughtco.com
What a Double Bond Means in Chemistry Why Are Double Bonds Easier To Break In this section, you will learn. But it gets more complicated. Branching decreases melting point and boiling point. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely. Why Are Double Bonds Easier To Break.
From phys.org
Breaking bonds Doublehelix unzipping reveals DNA physics Why Are Double Bonds Easier To Break But it gets more complicated. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. Look at these three examples of branched hexane derivatives (with. Yes, we're breaking the double bond in cyclohexene. A bond’s strength describes how strongly each atom is joined to. Why Are Double Bonds Easier To Break.
From www.slideserve.com
PPT Chapter 22 Chemical Bonding PowerPoint Presentation, free Why Are Double Bonds Easier To Break Look at these three examples of branched hexane derivatives (with. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond.. Why Are Double Bonds Easier To Break.
From www.youtube.com
LIVE I Double Bond Equivalent I Double Bond Equivalent of Benzene I Why Are Double Bonds Easier To Break Branching decreases melting point and boiling point. Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also. But it gets more complicated. Look at these three examples of branched hexane derivatives (with. In chemistry, a double bond is. Why Are Double Bonds Easier To Break.
From www.youtube.com
द्विबंध Double Bond त्रि बंध Triple Bond 11th Chemistry Why Are Double Bonds Easier To Break In this section, you will learn. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. Yes, we're breaking the double bond in cyclohexene. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond. Why Are Double Bonds Easier To Break.
From fabalabse.com
What are the 3 common bonds? Leia aqui What are the 3 types of bonds Why Are Double Bonds Easier To Break But it gets more complicated. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Energy required to. Why Are Double Bonds Easier To Break.
From www.numerade.com
SOLVEDWhat is (a) A single bond? (b) A double bond? (c) A triple bond? Why Are Double Bonds Easier To Break Branching decreases melting point and boiling point. Energy required to break double bond is 614 j while in breaking single bond is 349 j, thus the energy to break double bond is more. But it gets more complicated. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds.. Why Are Double Bonds Easier To Break.
From askfilo.com
Covalent bond b) Electrovalent or ionic bond C) Double bond d) none of t.. Why Are Double Bonds Easier To Break In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Look at these three examples of branched hexane derivatives (with. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. A bond’s strength describes. Why Are Double Bonds Easier To Break.
From slideplayer.com
Single, Double, and Triple Bonds Covalent Bonds. ppt download Why Are Double Bonds Easier To Break The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. Look at these three examples of branched hexane derivatives (with. Yes, we're breaking the double bond in cyclohexene. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required. Why Are Double Bonds Easier To Break.
From slideplayer.com
The intensity of the M+. ion is larger for the linear chain than the Why Are Double Bonds Easier To Break In this section, you will learn. Yes, we're breaking the double bond in cyclohexene. The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond.. Why Are Double Bonds Easier To Break.
From www.numerade.com
SOLVED 'Dlscuss the formation of single bond, double bonds and triple Why Are Double Bonds Easier To Break The shorter bond length in double bonds results in increased bond strength, making them more difficult to break compared to single bonds. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. But it gets more complicated. Branching decreases melting point and boiling point. Energy required. Why Are Double Bonds Easier To Break.
From www.chemistrystudent.com
Alkenes (ALevel) ChemistryStudent Why Are Double Bonds Easier To Break In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Look at these three examples of branched hexane derivatives (with. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the. Why Are Double Bonds Easier To Break.