How To Calculate Diameter Nucleus . Binding energy is very important: In previous discussions of rutherford’s scattering experiments, a light. The radius of nuclei depends on the nucleon number, a of the atom. By shooting alpha particles of kinetic energy 5.5. Binding energy is the energy required to split a nucleus into its constituents. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. Gives information on forces between nucleons. This makes sense because as more nucleons are added to a nucleus, more space is. The diameter of the nucleus, d, can be found using. The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. Let's derive an expression for the nuclear radius and calculate a numerical value for the.
from www.cyberphysics.co.uk
We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. Binding energy is very important: The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. The diameter of the nucleus, d, can be found using. By shooting alpha particles of kinetic energy 5.5. Binding energy is the energy required to split a nucleus into its constituents. This makes sense because as more nucleons are added to a nucleus, more space is. The radius of nuclei depends on the nucleon number, a of the atom. Gives information on forces between nucleons. Let's derive an expression for the nuclear radius and calculate a numerical value for the.
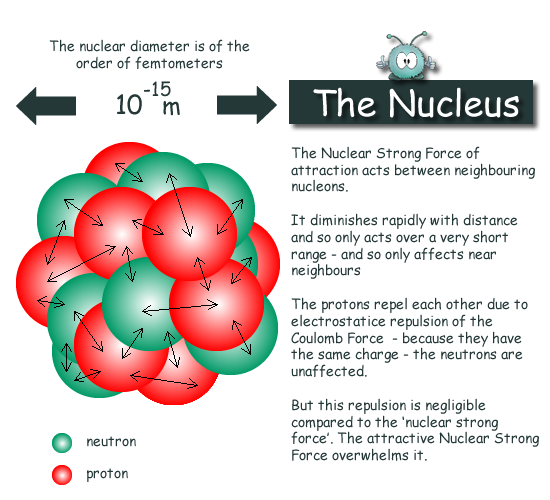
The nucleus
How To Calculate Diameter Nucleus Gives information on forces between nucleons. Gives information on forces between nucleons. Binding energy is very important: The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. The diameter of the nucleus, d, can be found using. In previous discussions of rutherford’s scattering experiments, a light. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. Binding energy is the energy required to split a nucleus into its constituents. The radius of nuclei depends on the nucleon number, a of the atom. This makes sense because as more nucleons are added to a nucleus, more space is. Let's derive an expression for the nuclear radius and calculate a numerical value for the. By shooting alpha particles of kinetic energy 5.5.
From www.cyberphysics.co.uk
The nucleus How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. The radius of nuclei depends on the nucleon number, a of the atom. In previous discussions of rutherford’s scattering experiments, a light. Binding energy is very important: This makes sense because as more nucleons are added to a nucleus, more space is. We can calculate the size. How To Calculate Diameter Nucleus.
From www.numerade.com
SOLVED You are observing cheek epithelial cells using the 40x How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. The diameter of the nucleus, d, can be found using. By shooting alpha particles of kinetic energy 5.5. This makes sense because as more nucleons are added to a nucleus, more space is. The radius of nuclei depends on the nucleon number, a of the atom. In. How To Calculate Diameter Nucleus.
From www.youtube.com
NUCLEAR DENSITY / HOW TO CALCULATE THE DENSITY OF A NUCLEUS YouTube How To Calculate Diameter Nucleus The radius of nuclei depends on the nucleon number, a of the atom. In previous discussions of rutherford’s scattering experiments, a light. By shooting alpha particles of kinetic energy 5.5. Binding energy is very important: We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. Binding energy is the energy required. How To Calculate Diameter Nucleus.
From byjus.com
The certainty in velocity of an electron present in the nucleus of How To Calculate Diameter Nucleus Binding energy is very important: The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. By shooting alpha particles of kinetic energy 5.5. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. The radius of nuclei. How To Calculate Diameter Nucleus.
From www.numerade.com
SOLVEDIf someone wanted to build a scale model of the atom with a How To Calculate Diameter Nucleus Binding energy is very important: The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. Let's derive an expression for the nuclear radius and calculate a numerical value for the. Gives information on forces between nucleons. Binding energy is the energy required to split a nucleus. How To Calculate Diameter Nucleus.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definitions.. and more How To Calculate Diameter Nucleus The radius of nuclei depends on the nucleon number, a of the atom. Gives information on forces between nucleons. Binding energy is very important: Binding energy is the energy required to split a nucleus into its constituents. This makes sense because as more nucleons are added to a nucleus, more space is. The angle θ, where the intensity is a. How To Calculate Diameter Nucleus.
From brainly.com
Which is larger the diameter of a nucleus or the diameter of a protron How To Calculate Diameter Nucleus By shooting alpha particles of kinetic energy 5.5. Let's derive an expression for the nuclear radius and calculate a numerical value for the. Binding energy is the energy required to split a nucleus into its constituents. In previous discussions of rutherford’s scattering experiments, a light. The angle θ, where the intensity is a minimum, can be explained by assuming that. How To Calculate Diameter Nucleus.
From www.numerade.com
SOLVEDA proton is confined within an atomic nucleus of diameter 4 fm(1 How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. The diameter of the nucleus, d, can be found using. The radius of nuclei depends on the nucleon number, a of the atom. Binding energy is very important:. How To Calculate Diameter Nucleus.
From physics.stackexchange.com
quantum mechanics Can Heisenberg's uncertainty principle be used to How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. Let's derive an expression for the nuclear radius and calculate a numerical value for the. The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. This makes sense because as more nucleons are. How To Calculate Diameter Nucleus.
From cegziosz.blob.core.windows.net
How To Compute Diameter From Circumference at Wanda Christofferso blog How To Calculate Diameter Nucleus The diameter of the nucleus, d, can be found using. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. Let's derive an expression for the nuclear radius and calculate a numerical value for the. Binding energy is the energy required to split a nucleus into its constituents. This makes sense. How To Calculate Diameter Nucleus.
From www.youtube.com
How to Calculate the Diameter of a Circle YouTube How To Calculate Diameter Nucleus Gives information on forces between nucleons. Let's derive an expression for the nuclear radius and calculate a numerical value for the. The diameter of the nucleus, d, can be found using. The radius of nuclei depends on the nucleon number, a of the atom. Binding energy is the energy required to split a nucleus into its constituents. This makes sense. How To Calculate Diameter Nucleus.
From byjus.com
A 24.6 MeV alpha particle moves towards gold nucleus (atomic number 79 How To Calculate Diameter Nucleus The diameter of the nucleus, d, can be found using. Binding energy is very important: This makes sense because as more nucleons are added to a nucleus, more space is. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. The angle θ, where the intensity is a minimum, can be. How To Calculate Diameter Nucleus.
From www.slideserve.com
PPT Nuclear Radius PowerPoint Presentation, free download ID6628861 How To Calculate Diameter Nucleus By shooting alpha particles of kinetic energy 5.5. Gives information on forces between nucleons. Let's derive an expression for the nuclear radius and calculate a numerical value for the. Binding energy is the energy required to split a nucleus into its constituents. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha. How To Calculate Diameter Nucleus.
From www.numerade.com
SOLVED Use the uncertainty principle to show that if an electron were How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. The radius of nuclei depends on the nucleon number, a of the atom. The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. Binding energy is very important: In previous discussions of rutherford’s. How To Calculate Diameter Nucleus.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent How To Calculate Diameter Nucleus Binding energy is very important: Let's derive an expression for the nuclear radius and calculate a numerical value for the. The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. The diameter of the nucleus, d, can be found using. Binding energy is the energy required. How To Calculate Diameter Nucleus.
From www.toppr.com
Find the binding energy of a nucleus consisting of equal numbers of How To Calculate Diameter Nucleus Let's derive an expression for the nuclear radius and calculate a numerical value for the. Binding energy is very important: The diameter of the nucleus, d, can be found using. The radius of nuclei depends on the nucleon number, a of the atom. We can calculate the size of the nucleus, by obtaining the point of closest approach of an. How To Calculate Diameter Nucleus.
From www.sciencefacts.net
Cell Nucleus Definition, Structure, & Function, with Diagram How To Calculate Diameter Nucleus Binding energy is very important: This makes sense because as more nucleons are added to a nucleus, more space is. The diameter of the nucleus, d, can be found using. Gives information on forces between nucleons. Let's derive an expression for the nuclear radius and calculate a numerical value for the. The radius of nuclei depends on the nucleon number,. How To Calculate Diameter Nucleus.
From www.slideserve.com
PPT Chapter Three PowerPoint Presentation, free download ID4330951 How To Calculate Diameter Nucleus We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. Gives information on forces between nucleons. Binding energy is the energy required to split a nucleus into its constituents. This makes sense because as more nucleons are added to a nucleus, more space is. In previous discussions of rutherford’s scattering experiments,. How To Calculate Diameter Nucleus.
From byjus.com
radius of a particular nucleus is calculated by projection of alpha How To Calculate Diameter Nucleus Binding energy is very important: By shooting alpha particles of kinetic energy 5.5. The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. In previous discussions of rutherford’s scattering experiments, a light. The diameter of the nucleus, d, can be found using. We can calculate the. How To Calculate Diameter Nucleus.
From www.youtube.com
How to calculate the RADIUS, DIAMETER and the CIRCUMFERENCE of a circle How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. The radius of nuclei depends on the nucleon number, a of the atom. In previous discussions of rutherford’s scattering experiments, a light. Let's derive an expression for the nuclear radius and calculate a numerical value for the. The angle θ, where the intensity is a minimum, can. How To Calculate Diameter Nucleus.
From www.shutterstock.com
How Measure Atomic Radius Distance Between 스톡 벡터(로열티 프리) 1482769742 How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. Let's derive an expression for the nuclear radius and calculate a numerical value for the. Gives information on forces between nucleons. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. This makes sense because as more nucleons. How To Calculate Diameter Nucleus.
From www.youtube.com
For a hydrogen atom in its ground state, use the Bohr model to compute How To Calculate Diameter Nucleus By shooting alpha particles of kinetic energy 5.5. The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. The diameter of the nucleus, d, can be found using. In previous discussions of rutherford’s scattering experiments, a light. The radius of nuclei depends on the nucleon number,. How To Calculate Diameter Nucleus.
From www.numerade.com
SOLVED An atom has a diameter of 3.50 Ã… and the nucleus of that atom How To Calculate Diameter Nucleus The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. Let's derive an expression for the nuclear radius and calculate a numerical value for the. Binding energy is very important: The diameter of the nucleus, d, can be found using. We can calculate the size of. How To Calculate Diameter Nucleus.
From www.chegg.com
Solved An atom has a diameter of 5.00 Å and the nucleus of How To Calculate Diameter Nucleus This makes sense because as more nucleons are added to a nucleus, more space is. The diameter of the nucleus, d, can be found using. Binding energy is the energy required to split a nucleus into its constituents. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. In previous discussions. How To Calculate Diameter Nucleus.
From www.numerade.com
SOLVED The diameter of the hydrogen nucleus is 2.5 × 10^15m, and the How To Calculate Diameter Nucleus Let's derive an expression for the nuclear radius and calculate a numerical value for the. Binding energy is the energy required to split a nucleus into its constituents. Binding energy is very important: By shooting alpha particles of kinetic energy 5.5. In previous discussions of rutherford’s scattering experiments, a light. The diameter of the nucleus, d, can be found using.. How To Calculate Diameter Nucleus.
From www.cuemath.com
Diameter of Circle Definition, Formula, Examples & Worksheets Cuemath How To Calculate Diameter Nucleus This makes sense because as more nucleons are added to a nucleus, more space is. Binding energy is the energy required to split a nucleus into its constituents. By shooting alpha particles of kinetic energy 5.5. Gives information on forces between nucleons. The diameter of the nucleus, d, can be found using. Binding energy is very important: The angle θ,. How To Calculate Diameter Nucleus.
From www.youtube.com
Nuclear radius in Hindi radius of nucleus Formula nuclear radius How To Calculate Diameter Nucleus By shooting alpha particles of kinetic energy 5.5. The radius of nuclei depends on the nucleon number, a of the atom. Binding energy is very important: The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. Gives information on forces between nucleons. Let's derive an expression. How To Calculate Diameter Nucleus.
From ar.inspiredpencil.com
Measuring Atomic Radius How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. The diameter of the nucleus, d, can be found using. In previous discussions of rutherford’s scattering experiments, a light. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. The radius of nuclei depends on the nucleon number,. How To Calculate Diameter Nucleus.
From www.toppr.com
If nucleus and atom are considered as perfect spheres with the How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. The diameter of the nucleus, d, can be found using. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. The radius of nuclei depends on the nucleon number, a of the atom. Gives information on forces between. How To Calculate Diameter Nucleus.
From www.wikihow.com
How to Find the Diameter of a Circle wikiHow How To Calculate Diameter Nucleus Binding energy is the energy required to split a nucleus into its constituents. By shooting alpha particles of kinetic energy 5.5. The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. This makes sense because as more nucleons are added to a nucleus, more space is.. How To Calculate Diameter Nucleus.
From www.numerade.com
SOLVED 2) Calculate the minimum energy ofa neutron in a How To Calculate Diameter Nucleus In previous discussions of rutherford’s scattering experiments, a light. Binding energy is the energy required to split a nucleus into its constituents. Let's derive an expression for the nuclear radius and calculate a numerical value for the. Binding energy is very important: This makes sense because as more nucleons are added to a nucleus, more space is. The radius of. How To Calculate Diameter Nucleus.
From cemjljnl.blob.core.windows.net
How To Calculate Effective Diameter at Elizabeth Tejada blog How To Calculate Diameter Nucleus Binding energy is very important: The radius of nuclei depends on the nucleon number, a of the atom. In previous discussions of rutherford’s scattering experiments, a light. Binding energy is the energy required to split a nucleus into its constituents. This makes sense because as more nucleons are added to a nucleus, more space is. We can calculate the size. How To Calculate Diameter Nucleus.
From exobehtve.blob.core.windows.net
How To Calculate The Diameter Of A Gold Nucleus In Nm at Daniel Hedlund How To Calculate Diameter Nucleus This makes sense because as more nucleons are added to a nucleus, more space is. Binding energy is very important: We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. Gives information on forces between nucleons. The angle θ, where the intensity is a minimum, can be explained by assuming that. How To Calculate Diameter Nucleus.
From www.slideserve.com
PPT Lecture 4. Chapter 2. Structure of the Atom (Contd.) PowerPoint How To Calculate Diameter Nucleus The radius of nuclei depends on the nucleon number, a of the atom. We can calculate the size of the nucleus, by obtaining the point of closest approach of an alpha particle. The diameter of the nucleus, d, can be found using. Gives information on forces between nucleons. In previous discussions of rutherford’s scattering experiments, a light. By shooting alpha. How To Calculate Diameter Nucleus.
From materialmediapatines.z21.web.core.windows.net
How To Find Out The Diameter Of A Circle How To Calculate Diameter Nucleus This makes sense because as more nucleons are added to a nucleus, more space is. Gives information on forces between nucleons. The angle θ, where the intensity is a minimum, can be explained by assuming that the electrons have diffracted around a spherical object (i.e. By shooting alpha particles of kinetic energy 5.5. We can calculate the size of the. How To Calculate Diameter Nucleus.