Standard Reduction Potential Questions . Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. What does the standard reduction potential measure? Calculate cell potentials and predict redox spontaneity using. Use standard reduction potentials to determine the better oxidizing or. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? What conditions must be met for a potential Use standard reduction potentials to. Interpret electrode potentials in terms of relative oxidant and reductant strengths. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire.
from www.chegg.com
The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. What does the standard reduction potential measure? Use standard reduction potentials to determine the better oxidizing or. What conditions must be met for a potential Calculate cell potentials and predict redox spontaneity using. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials.
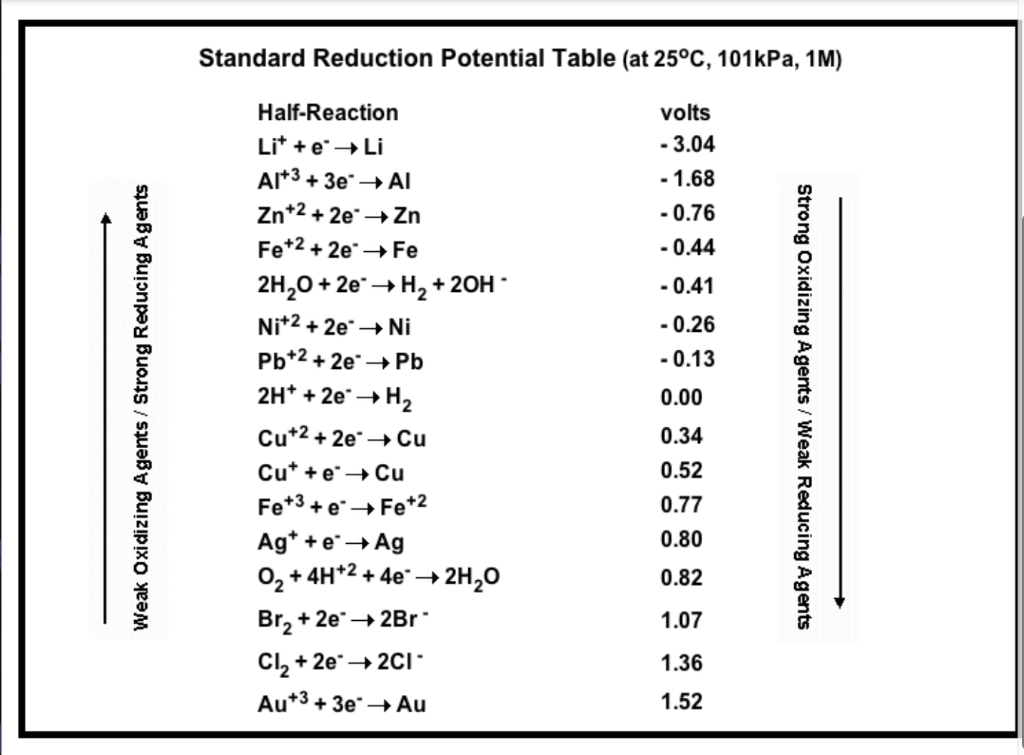
Solved Standard Reduction Potential Table (at 25°C, 101kPa,
Standard Reduction Potential Questions The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the better oxidizing or. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? Interpret electrode potentials in terms of relative oxidant and reductant strengths. What conditions must be met for a potential The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Calculate cell potentials and predict redox spontaneity using. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. Use standard reduction potentials to. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. What does the standard reduction potential measure?
From www.chegg.com
Solved Given these standard reduction potentials at 25 Standard Reduction Potential Questions What does the standard reduction potential measure? By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency. Standard Reduction Potential Questions.
From www.bartleby.com
Answered Standard reduction potentials for… bartleby Standard Reduction Potential Questions Interpret electrode potentials in terms of relative oxidant and reductant strengths. What conditions must be met for a potential Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. By convention, all tabulated values of standard electrode. Standard Reduction Potential Questions.
From www.chegg.com
Solved A chemist designs a galvanic cell that uses these two Standard Reduction Potential Questions What does the standard reduction potential measure? Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. Calculate cell potentials and predict redox spontaneity using. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? Use standard reduction. Standard Reduction Potential Questions.
From www.chegg.com
Solved Use the standard reduction potential table and the Standard Reduction Potential Questions Interpret electrode potentials in terms of relative oxidant and reductant strengths. What does the standard reduction potential measure? Use standard reduction potentials to determine the better oxidizing or. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Calculate cell potentials and predict redox spontaneity using. What are the differences between the standard reduction potential. Standard Reduction Potential Questions.
From www.toppr.com
The standard reduction potential the reaction Ag^{+}+e^{}rightarrow Standard Reduction Potential Questions Calculate cell potentials and predict redox spontaneity using. Use standard reduction potentials to. What does the standard reduction potential measure? By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? Interpret electrode potentials in terms of. Standard Reduction Potential Questions.
From www.chegg.com
Solved Standard Reduction Potentials (V) in Aqueous Standard Reduction Potential Questions What does the standard reduction potential measure? What conditions must be met for a potential Calculate cell potentials and predict redox spontaneity using. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to. By convention, all tabulated values of standard electrode potentials. Standard Reduction Potential Questions.
From www.chegg.com
Solved Standard Reduction Potential Table (at 25°C, 101kPa, Standard Reduction Potential Questions Use standard reduction potentials to determine the better oxidizing or. Use standard reduction potentials to. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related?. Standard Reduction Potential Questions.
From www.chegg.com
Solved Using data from the Standard Reduction Potentials Standard Reduction Potential Questions Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? Standard reduction potential. Standard Reduction Potential Questions.
From www.chegg.com
Solved using the standard reduction potentials Determine if Standard Reduction Potential Questions Calculate cell potentials and predict redox spontaneity using. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to. Use standard reduction potentials to determine the better oxidizing. Standard Reduction Potential Questions.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Questions By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What. Standard Reduction Potential Questions.
From www.toppr.com
The standard reduction potential the half cell NO^_3(aq)+2H^+(aq Standard Reduction Potential Questions By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Use standard reduction potentials to. What does the standard reduction potential measure? The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Interpret electrode potentials in terms of relative oxidant and reductant. Standard Reduction Potential Questions.
From www.chegg.com
Solved A chemist designs a galvanic cell that uses these two Standard Reduction Potential Questions What does the standard reduction potential measure? By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? What conditions must be met for a potential The standard reduction potential, also known as the standard electrode potential,. Standard Reduction Potential Questions.
From www.chegg.com
Solved 5. (a) Use the standard reduction potentials at 25° C Standard Reduction Potential Questions Use standard reduction potentials to determine the better oxidizing or. Use standard reduction potentials to. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What are the differences between the standard reduction potential and standard. Standard Reduction Potential Questions.
From www.chegg.com
Solved Using standard reduction potentials from the ALEKS Standard Reduction Potential Questions Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. What does the standard reduction potential measure? What conditions must be met for a potential Calculate cell potentials and predict redox spontaneity using. By convention, all tabulated values of standard electrode potentials are listed as standard reduction. Standard Reduction Potential Questions.
From www.toppr.com
The standard reduction potential for the half cell NO3^(aq) + 2H Standard Reduction Potential Questions By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the better oxidizing or. Use standard reduction potentials to. Standard reduction potential is a measure of. Standard Reduction Potential Questions.
From www.chegg.com
Solved 8. What is the standard reduction potential (EⓇ) for Standard Reduction Potential Questions Use standard reduction potentials to determine the better oxidizing or. Use standard reduction potentials to. What conditions must be met for a potential Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. What does the standard reduction potential measure? By convention, all tabulated values of standard. Standard Reduction Potential Questions.
From www.chegg.com
Solved Selective Reduction The standard reduction potential Standard Reduction Potential Questions By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. What does the standard reduction potential measure? What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? Use standard reduction potentials to. Calculate cell potentials. Standard Reduction Potential Questions.
From www.chegg.com
Solved What is the standard reduction potential (x) for the Standard Reduction Potential Questions What conditions must be met for a potential The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Standard reduction potential is a measure of the tendency. Standard Reduction Potential Questions.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Questions Interpret electrode potentials in terms of relative oxidant and reductant strengths. Use standard reduction potentials to. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. What are the differences between the. Standard Reduction Potential Questions.
From www.chegg.com
Solved Table 20.1 Standard Reduction Potentials for Several Standard Reduction Potential Questions By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Use standard reduction potentials to determine the better oxidizing or. Calculate cell potentials and predict redox spontaneity using. What does the standard reduction potential measure? Interpret electrode potentials in terms of relative oxidant and reductant strengths. What are the differences between the standard reduction potential. Standard Reduction Potential Questions.
From byjus.com
The standard reduction potential of the reaction at 25^° C ,Н2О + e Standard Reduction Potential Questions What does the standard reduction potential measure? Use standard reduction potentials to determine the better oxidizing or. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Interpret. Standard Reduction Potential Questions.
From www.toppr.com
The standard reduction potential for the half cell reaction, Cl2 + 2e Standard Reduction Potential Questions Use standard reduction potentials to. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the better oxidizing or. Calculate cell potentials and predict redox spontaneity using. Interpret electrode potentials in terms of relative oxidant and reductant strengths. What does the. Standard Reduction Potential Questions.
From www.chegg.com
Solved TABLE 20.1 Standard Reduction Potentials in Water at Standard Reduction Potential Questions Use standard reduction potentials to determine the better oxidizing or. Use standard reduction potentials to. What does the standard reduction potential measure? Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What are the differences. Standard Reduction Potential Questions.
From www.bartleby.com
Answered Selected Standard Reduction Potentials… bartleby Standard Reduction Potential Questions Use standard reduction potentials to. What does the standard reduction potential measure? Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Calculate cell potentials and predict redox spontaneity using. What are the differences between the. Standard Reduction Potential Questions.
From www.chegg.com
Solved Refer to the table of standard reduction potentials Standard Reduction Potential Questions The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What conditions must be met for a potential Use standard reduction potentials to determine the better oxidizing or. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Use standard reduction potentials to. What are the. Standard Reduction Potential Questions.
From www.chegg.com
Solved Use the standard reduction potentials located in the Standard Reduction Potential Questions Calculate cell potentials and predict redox spontaneity using. Use standard reduction potentials to. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What does the standard reduction potential measure? What conditions must be met for a potential Standard reduction potential is a measure of the tendency. Standard Reduction Potential Questions.
From www.chegg.com
Solved A chemist designs a galvanic cell that uses these two Standard Reduction Potential Questions Interpret electrode potentials in terms of relative oxidant and reductant strengths. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Calculate cell potentials and predict redox spontaneity using. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What does the. Standard Reduction Potential Questions.
From www.chegg.com
Solved TABLE 20.1 Standard Reduction Potentials for Several Standard Reduction Potential Questions Calculate cell potentials and predict redox spontaneity using. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to. What. Standard Reduction Potential Questions.
From www.toppr.com
The standard reduction potential for the half cell NO3^(aq) + 2H Standard Reduction Potential Questions The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? Interpret electrode potentials in terms of relative oxidant and reductant strengths. Standard reduction potential is a measure of. Standard Reduction Potential Questions.
From www.toppr.com
The standard reduction potential Cu2+/Cu is +0.34 V. What will be the Standard Reduction Potential Questions Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard reduction potential, also known as the standard electrode potential,. Standard Reduction Potential Questions.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Questions What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the better oxidizing or. By convention, all tabulated values of standard electrode. Standard Reduction Potential Questions.
From www.chegg.com
Solved A chemist designs a galvanic cell that uses these two Standard Reduction Potential Questions Calculate cell potentials and predict redox spontaneity using. Use standard reduction potentials to. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Standard reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced, represented by the standard. The standard reduction potential, also known as the standard. Standard Reduction Potential Questions.
From www.toppr.com
If for an electrode, the standard reduction potential is negative then Standard Reduction Potential Questions The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to. What does the standard reduction potential measure? Interpret electrode potentials in terms of relative oxidant and reductant strengths. Calculate cell potentials and predict redox spontaneity using. Standard reduction potential is a measure. Standard Reduction Potential Questions.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Questions What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? Use standard reduction potentials to determine the better oxidizing or. Use standard reduction potentials to. Calculate cell potentials and predict redox spontaneity using. By convention, all tabulated values of standard electrode potentials are listed as standard reduction potentials. Standard reduction potential. Standard Reduction Potential Questions.
From www.chegg.com
Solved Using standard reduction potentials from the ALEKS Standard Reduction Potential Questions What conditions must be met for a potential What are the differences between the standard reduction potential and standard oxidation potential, and how are the two related? The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Standard reduction potential is a measure of the tendency of. Standard Reduction Potential Questions.