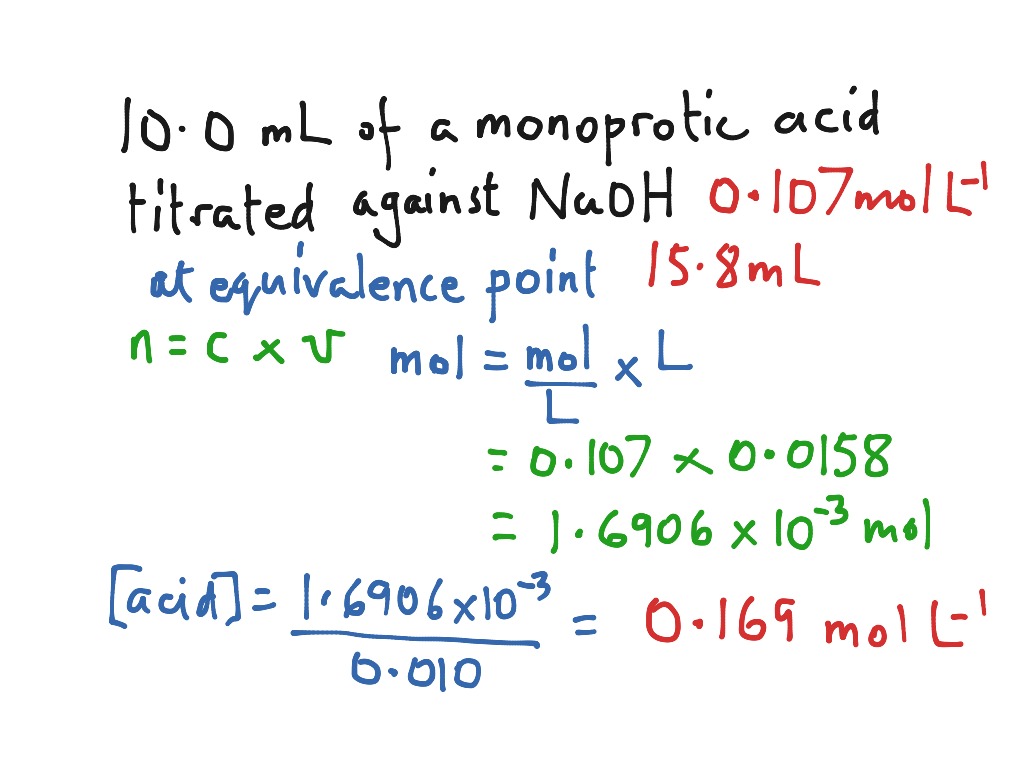
Titration Value Calculation . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. perform and interpret titration calculations. our titration calculator will help you never have to ask how do i calculate titrations?.
from www.showme.com
The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. perform and interpret titration calculations. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). our titration calculator will help you never have to ask how do i calculate titrations?.
Titration calculation Science, Chemistry, Physical Chemistry ShowMe
Titration Value Calculation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. our titration calculator will help you never have to ask how do i calculate titrations?. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. perform and interpret titration calculations. A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Value Calculation perform and interpret titration calculations. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). . Titration Value Calculation.
From mungfali.com
Acid Base Titration Calculation Titration Value Calculation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. perform and interpret titration calculations. A process for determining the unknown concentration of a reactant by. Titration Value Calculation.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Value Calculation perform and interpret titration calculations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Titration Value Calculation.
From mungfali.com
Acid Base Titration Calculation Titration Value Calculation A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). perform and interpret titration calculations. our titration calculator will. Titration Value Calculation.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Value Calculation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. our titration calculator will help you never have to ask how do i calculate titrations?. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is a volumetric. Titration Value Calculation.
From exohtntjr.blob.core.windows.net
How To Calculate Titration Value at Eric Whitlow blog Titration Value Calculation a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A process for determining the unknown concentration of a reactant. Titration Value Calculation.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Value Calculation perform and interpret titration calculations. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. The basic process involves adding. Titration Value Calculation.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog Titration Value Calculation The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. At the equivalence point in a neutralization, the moles of acid. Titration Value Calculation.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Value Calculation our titration calculator will help you never have to ask how do i calculate titrations?. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A process for determining the unknown. Titration Value Calculation.
From www.youtube.com
Titration Calculations YouTube Titration Value Calculation A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. our titration calculator will help you never have to ask how do i calculate titrations?. perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration Value Calculation.
From www.youtube.com
Total Alkalinity Titration Method and Calculations YouTube Titration Value Calculation perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. our titration calculator will. Titration Value Calculation.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Value Calculation a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. At the equivalence point in a neutralization, the moles of acid are. Titration Value Calculation.
From www.youtube.com
28 TITRATION 1 Experiment , Calculation HSC Chemistry YouTube Titration Value Calculation a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves. Titration Value Calculation.
From www.researchgate.net
qPCR analysis for lentivirus titration. Representative triplicate Cq Titration Value Calculation a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. . Titration Value Calculation.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Value Calculation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. perform and interpret titration calculations. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is a volumetric technique in which a solution. Titration Value Calculation.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration Value Calculation perform and interpret titration calculations. our titration calculator will help you never have to ask how do i calculate titrations?. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is a laboratory technique used to precisely measure molar concentration of an unknown. Titration Value Calculation.
From www.youtube.com
titration curve pH calculations YouTube Titration Value Calculation perform and interpret titration calculations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. a titration is carried out for. Titration Value Calculation.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Value Calculation our titration calculator will help you never have to ask how do i calculate titrations?. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. Titration Value Calculation.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Value Calculation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. perform and interpret titration calculations. our titration calculator will help you never have to ask. Titration Value Calculation.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Value Calculation a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. perform and interpret titration calculations. A process for determining the. Titration Value Calculation.
From www.youtube.com
Excel Tutorial 2 Titration Analysis YouTube Titration Value Calculation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. The basic process. Titration Value Calculation.
From www.youtube.com
Calculations with titrations 3 YouTube Titration Value Calculation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. our titration calculator will help you never have to ask how do i calculate titrations?. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A process for determining the unknown. Titration Value Calculation.
From dxowlwwmg.blob.core.windows.net
Titration Experiment Titration Results Table at Barbara Dorman blog Titration Value Calculation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. our titration calculator will help you never have to ask how do i calculate titrations?. A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. The basic process involves adding. Titration Value Calculation.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Value Calculation a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A process for determining the unknown concentration of a reactant by. Titration Value Calculation.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Value Calculation A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. perform and interpret titration calculations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Titration Value Calculation.
From www.youtube.com
13 Titration Sample Calculation YouTube Titration Value Calculation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). our titration calculator will help you never have to ask how. Titration Value Calculation.
From www.youtube.com
Titration Calculations YouTube Titration Value Calculation our titration calculator will help you never have to ask how do i calculate titrations?. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. perform and interpret titration calculations. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a. Titration Value Calculation.
From cwsimons.com
How to Draw Titration Curves of Amino Acids Food Science Toolbox Titration Value Calculation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Titration Value Calculation.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Value Calculation a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. At the equivalence point in a neutralization, the. Titration Value Calculation.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Value Calculation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent.. Titration Value Calculation.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Value Calculation The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of. Titration Value Calculation.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Value Calculation our titration calculator will help you never have to ask how do i calculate titrations?. A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration. Titration Value Calculation.
From www.scribd.com
Titration Class XI PDF Titration Value Calculation a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). our titration calculator will help you never have to ask how do i calculate titrations?. A titration is a laboratory technique used to precisely measure molar concentration of an. Titration Value Calculation.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID4273257 Titration Value Calculation perform and interpret titration calculations. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A process for determining the unknown concentration of a reactant by reacting it with a reactant of known concentration. a titration is a. Titration Value Calculation.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Value Calculation our titration calculator will help you never have to ask how do i calculate titrations?. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Titration Value Calculation.