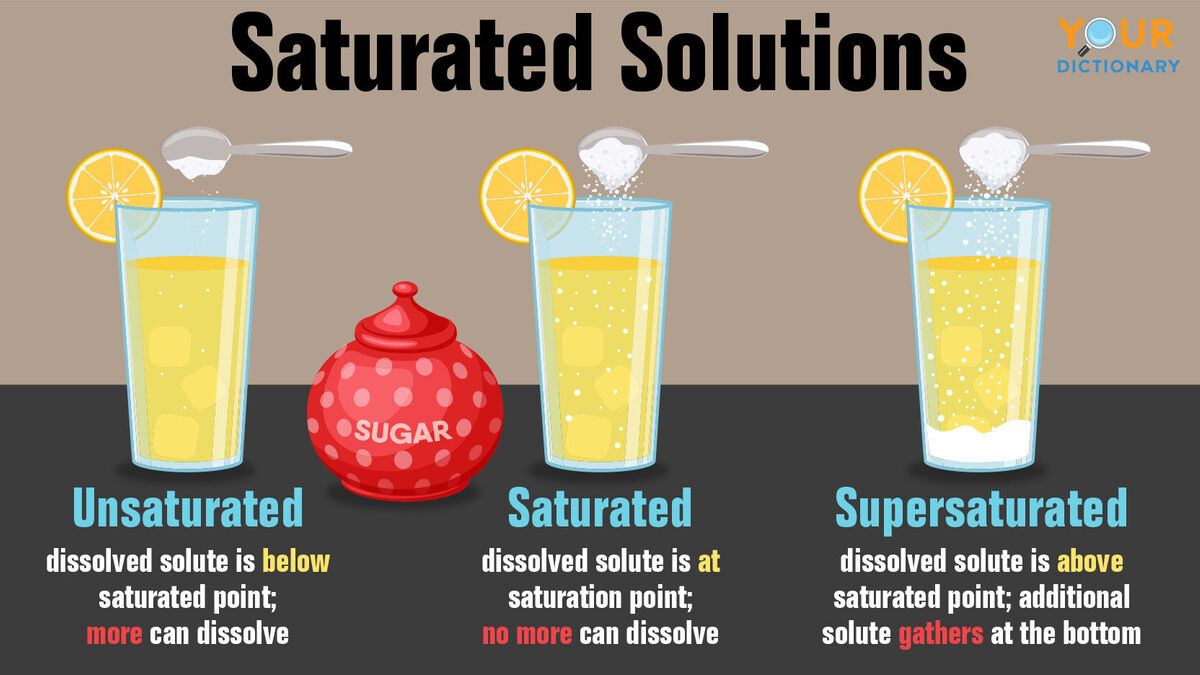
Unsaturated Solution Definition Quizlet . Solution containing all the dissolved solute. because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. What is a supersaturated solution? When a solvent can still dissolve a solute. A solution that contains more solute than it can theoretically hold at a given temperature. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. if the added solute dissolves, then the original solution was unsaturated. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated.
from ar.inspiredpencil.com
because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. What is a supersaturated solution? A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. if the added solute dissolves, then the original solution was unsaturated. When a solvent can still dissolve a solute. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Solution containing all the dissolved solute. A solution that contains more solute than it can theoretically hold at a given temperature.
Types Of Solutions Saturated Unsaturated And Supersaturated
Unsaturated Solution Definition Quizlet A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. What is a supersaturated solution? When a solvent can still dissolve a solute. A solution that contains more solute than it can theoretically hold at a given temperature. Solution containing all the dissolved solute. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. if the added solute dissolves, then the original solution was unsaturated.
From testbook.com
Unsaturated Solutions Definition, Preparation, Application Insights Unsaturated Solution Definition Quizlet When a solvent can still dissolve a solute. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. What is a supersaturated solution? Solution containing all the dissolved solute. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of. Unsaturated Solution Definition Quizlet.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated Unsaturated Solution Definition Quizlet Solution containing all the dissolved solute. because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. if the added solute dissolves, then the original solution was unsaturated. What is a supersaturated solution? When a solvent can still dissolve a solute. A solution that has been allowed to. Unsaturated Solution Definition Quizlet.
From ar.inspiredpencil.com
Unsaturated Solution Unsaturated Solution Definition Quizlet What is a supersaturated solution? study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. if the added solute dissolves, then the original solution was unsaturated. Solution containing all the dissolved solute. an unsaturated solution is a solution that contains less than the maximum amount of solute that. Unsaturated Solution Definition Quizlet.
From studymagiclemann.z19.web.core.windows.net
Saturated And Unsaturated Solutions Worksheet Unsaturated Solution Definition Quizlet because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. Solution containing all the dissolved solute. When a solvent can still dissolve a solute. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. A solution that contains. Unsaturated Solution Definition Quizlet.
From learningdbehrlichmann.z21.web.core.windows.net
Saturated And Unsaturated Solutions Worksheet Unsaturated Solution Definition Quizlet an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom. Unsaturated Solution Definition Quizlet.
From slidesharetrick.blogspot.com
What Is An Unsaturated Solution Quizlet slidesharetrick Unsaturated Solution Definition Quizlet an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. When a solvent can still dissolve a solute. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. A solution that contains more solute. Unsaturated Solution Definition Quizlet.
From www.slideserve.com
PPT Unit 7 Solution Chemistry Chapter 13 PowerPoint Presentation Unsaturated Solution Definition Quizlet A solution that contains more solute than it can theoretically hold at a given temperature. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. if the added solute dissolves, then the original solution was unsaturated. Solution containing all the dissolved solute. A solution that has been. Unsaturated Solution Definition Quizlet.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated Unsaturated Solution Definition Quizlet When a solvent can still dissolve a solute. if the added solute dissolves, then the original solution was unsaturated. A solution that contains more solute than it can theoretically hold at a given temperature. because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. A solution that. Unsaturated Solution Definition Quizlet.
From studyingworksheets.com
Saturated And Unsaturated Solutions Worksheet Studying Worksheets Unsaturated Solution Definition Quizlet because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. What is a supersaturated solution? if the added solute dissolves, then the original solution was unsaturated. A solution that contains more solute than it can theoretically hold at a given temperature. A solution that has been allowed. Unsaturated Solution Definition Quizlet.
From www.slideserve.com
PPT Chapter 7 Solutions PowerPoint Presentation, free download ID Unsaturated Solution Definition Quizlet an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. A solution that contains more solute than it can theoretically hold at a given temperature. if the added solute dissolves, then the original solution was unsaturated. study with quizlet and memorize flashcards containing terms like a. Unsaturated Solution Definition Quizlet.
From studylibraryklatsch.z22.web.core.windows.net
Saturated And Unsaturated Solutions Worksheet Unsaturated Solution Definition Quizlet Solution containing all the dissolved solute. if the added solute dissolves, then the original solution was unsaturated. because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of. Unsaturated Solution Definition Quizlet.
From slideplayer.com
Natural Sciences and Technology Grade 6 ppt download Unsaturated Solution Definition Quizlet Solution containing all the dissolved solute. When a solvent can still dissolve a solute. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. What is a supersaturated solution? A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container,. Unsaturated Solution Definition Quizlet.
From www.pinterest.com
Unsaturated Solution Easy Science Easy science, Solutions, Science Unsaturated Solution Definition Quizlet When a solvent can still dissolve a solute. if the added solute dissolves, then the original solution was unsaturated. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. A solution that contains more solute than it can theoretically hold at a given temperature. because the amount of. Unsaturated Solution Definition Quizlet.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated Unsaturated Solution Definition Quizlet Solution containing all the dissolved solute. What is a supersaturated solution? an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. if the added solute dissolves, then. Unsaturated Solution Definition Quizlet.
From edurev.in
what is difference between saturated, unsaturated and supersaturated Unsaturated Solution Definition Quizlet A solution that contains more solute than it can theoretically hold at a given temperature. Solution containing all the dissolved solute. What is a supersaturated solution? because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. study with quizlet and memorize flashcards containing terms like a saturated. Unsaturated Solution Definition Quizlet.
From www.youtube.com
Saturated , Unsaturated solution and Solubility Definition YouTube Unsaturated Solution Definition Quizlet because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. if the added solute dissolves, then the original solution was unsaturated. A solution that contains more solute. Unsaturated Solution Definition Quizlet.
From www.teachoo.com
Difference between Saturated and Unsaturated Solution Teachoo Unsaturated Solution Definition Quizlet study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. Solution containing all the dissolved solute. When a solvent can still dissolve a solute. What is a supersaturated solution? because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should. Unsaturated Solution Definition Quizlet.
From www.slideserve.com
PPT Review Next 3 slides PowerPoint Presentation, free download ID Unsaturated Solution Definition Quizlet A solution that contains more solute than it can theoretically hold at a given temperature. if the added solute dissolves, then the original solution was unsaturated. What is a supersaturated solution? Solution containing all the dissolved solute. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved.. Unsaturated Solution Definition Quizlet.
From www.slideserve.com
PPT Chapter 4 Solution Chemistry and the Hydrosphere PowerPoint Unsaturated Solution Definition Quizlet When a solvent can still dissolve a solute. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. if the added solute dissolves, then the. Unsaturated Solution Definition Quizlet.
From www.youtube.com
Identifying properties of unsaturated solutions YouTube Unsaturated Solution Definition Quizlet study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. if the added solute dissolves, then the original solution was unsaturated. What is a supersaturated solution? A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be. Unsaturated Solution Definition Quizlet.
From slidesharetrick.blogspot.com
What Is An Unsaturated Solution Quizlet slidesharetrick Unsaturated Solution Definition Quizlet if the added solute dissolves, then the original solution was unsaturated. What is a supersaturated solution? because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. A. Unsaturated Solution Definition Quizlet.
From www.scribd.com
Saturated and Unsaturated Solution (QRT) PDF Solubility Concentration Unsaturated Solution Definition Quizlet because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. When a solvent can still dissolve a solute. Solution containing all the dissolved solute. an unsaturated solution. Unsaturated Solution Definition Quizlet.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Unsaturated Solution Definition Quizlet A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. A solution that contains more solute than it can theoretically hold at a given temperature. because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should. Unsaturated Solution Definition Quizlet.
From byjus.com
54. explain unsaturated solution saturated solutions and supersaturated Unsaturated Solution Definition Quizlet A solution that contains more solute than it can theoretically hold at a given temperature. When a solvent can still dissolve a solute. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. because the amount of solute that is contained in a supersaturated solution. Unsaturated Solution Definition Quizlet.
From quizzmagicfarris.z21.web.core.windows.net
Saturated And Unsaturated Solutions Ppt Unsaturated Solution Definition Quizlet A solution that contains more solute than it can theoretically hold at a given temperature. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. Solution containing all the dissolved solute. What is a supersaturated solution? study with quizlet and memorize flashcards containing terms like. Unsaturated Solution Definition Quizlet.
From brainly.in
define unsaturated solution Brainly.in Unsaturated Solution Definition Quizlet Solution containing all the dissolved solute. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. because the amount of solute that is contained in a supersaturated. Unsaturated Solution Definition Quizlet.
From ar.inspiredpencil.com
Unsaturated Solution Unsaturated Solution Definition Quizlet Solution containing all the dissolved solute. because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. A solution that contains more solute than it can theoretically hold at a given temperature. an unsaturated solution is a solution that contains less than the maximum amount of solute that. Unsaturated Solution Definition Quizlet.
From www.slideserve.com
PPT Solutions and Solubility PowerPoint Presentation, free download Unsaturated Solution Definition Quizlet What is a supersaturated solution? Solution containing all the dissolved solute. because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. When a solvent can still. Unsaturated Solution Definition Quizlet.
From www.youtube.com
Define Unsaturated Solution /Definition of Unsaturated Solution Unsaturated Solution Definition Quizlet Solution containing all the dissolved solute. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. When a solvent can still dissolve a solute. if. Unsaturated Solution Definition Quizlet.
From www.thoughtco.com
What Is an Unsaturated Solution in Chemistry? Unsaturated Solution Definition Quizlet Solution containing all the dissolved solute. What is a supersaturated solution? an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. When a solvent can still dissolve a solute. if the added solute dissolves, then the original solution was unsaturated. because the amount of solute that. Unsaturated Solution Definition Quizlet.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Unsaturated Solution Definition Quizlet because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. Solution containing all the dissolved solute. study with quizlet and memorize flashcards containing. Unsaturated Solution Definition Quizlet.
From quizlet.com
Mixtures and Solutions Diagram Quizlet Unsaturated Solution Definition Quizlet A solution that contains more solute than it can theoretically hold at a given temperature. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. What. Unsaturated Solution Definition Quizlet.
From slideplayer.com
Chapter 22 Solutions Lesson ppt download Unsaturated Solution Definition Quizlet A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. Solution containing all the dissolved solute. if the added solute dissolves, then the. Unsaturated Solution Definition Quizlet.
From printableuklanjalqb.z22.web.core.windows.net
Saturated And Unsaturated Solutions Worksheet Unsaturated Solution Definition Quizlet A solution that contains more solute than it can theoretically hold at a given temperature. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Solution containing all the dissolved solute. if the added solute dissolves, then the original solution was unsaturated. A solution that has been. Unsaturated Solution Definition Quizlet.
From study.com
Quiz & Worksheet Features of Unsaturated Solutions Unsaturated Solution Definition Quizlet because the amount of solute that is contained in a supersaturated solution exceeds the natural quantity of solute that should be. study with quizlet and memorize flashcards containing terms like a saturated solution is, a unsaturated solution is, a supersaturated. an unsaturated solution is a solution that contains less than the maximum amount of solute that is. Unsaturated Solution Definition Quizlet.