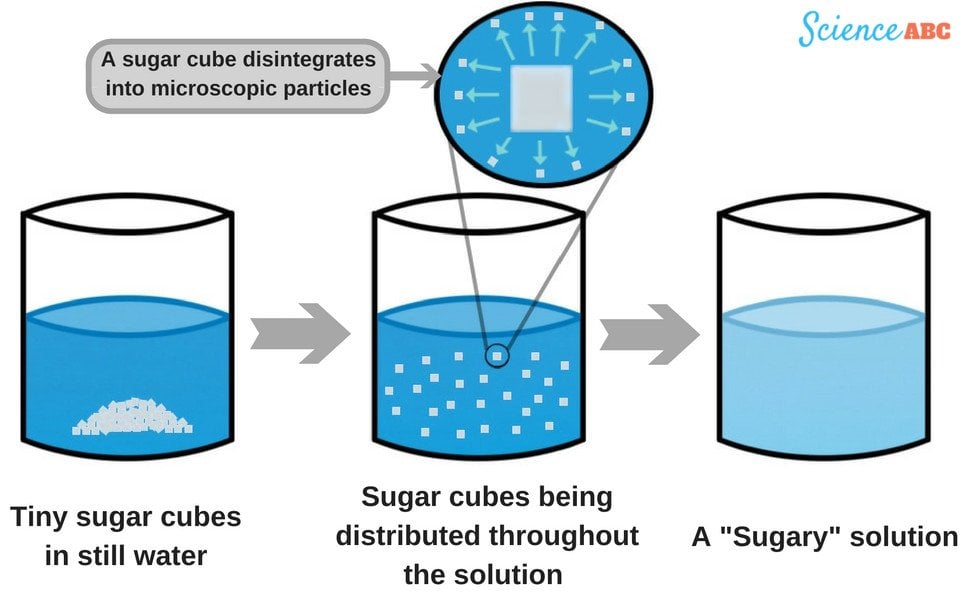
What Powder Dissolves In Water . Learn more about how materials react to water in this bbc bitesize ks1 science. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Nonpolar molecules, such as those found in grease. Water molecules in front of and behind the ions are not shown. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). As potassium chloride (kcl) dissolves in water, the ions are hydrated. why do some material disappear in water? Into a solvent, it is. The polar water molecules are attracted by the charges on the k + and cl − ions. water typically dissolves most ionic compounds and polar molecules. a solution is made when a.
from www.scienceabc.com
when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Into a solvent, it is. The polar water molecules are attracted by the charges on the k + and cl − ions. a solution is made when a. why do some material disappear in water? Nonpolar molecules, such as those found in grease. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). water typically dissolves most ionic compounds and polar molecules. Water molecules in front of and behind the ions are not shown. Learn more about how materials react to water in this bbc bitesize ks1 science.
Why Does Sugar Disappear When It Dissolves In Water? » ScienceABC
What Powder Dissolves In Water As potassium chloride (kcl) dissolves in water, the ions are hydrated. why do some material disappear in water? when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). a solution is made when a. Nonpolar molecules, such as those found in grease. Water molecules in front of and behind the ions are not shown. water typically dissolves most ionic compounds and polar molecules. Into a solvent, it is. As potassium chloride (kcl) dissolves in water, the ions are hydrated. Learn more about how materials react to water in this bbc bitesize ks1 science. The polar water molecules are attracted by the charges on the k + and cl − ions.
From www.youtube.com
Why Does This Powder Only Dissolve In Cold Water? YouTube What Powder Dissolves In Water Learn more about how materials react to water in this bbc bitesize ks1 science. Into a solvent, it is. As potassium chloride (kcl) dissolves in water, the ions are hydrated. a solution is made when a. Water molecules in front of and behind the ions are not shown. The polar water molecules are attracted by the charges on the. What Powder Dissolves In Water.
From ar.inspiredpencil.com
Solubility Science What Powder Dissolves In Water why do some material disappear in water? a solution is made when a. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Into a solvent, it is. The polar water molecules are attracted by the charges on the k + and cl − ions. Water. What Powder Dissolves In Water.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures What Powder Dissolves In Water The polar water molecules are attracted by the charges on the k + and cl − ions. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). why do some material disappear in water? Nonpolar molecules, such as those found in grease. water typically dissolves most ionic compounds and polar molecules. Water molecules. What Powder Dissolves In Water.
From userdatarheumatics.z21.web.core.windows.net
Chemical Equation For Salt Dissolved In Water What Powder Dissolves In Water Into a solvent, it is. As potassium chloride (kcl) dissolves in water, the ions are hydrated. The polar water molecules are attracted by the charges on the k + and cl − ions. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Water molecules in front of. What Powder Dissolves In Water.
From fphoto.photoshelter.com
science chemistry compound sulfur Fundamental Photographs The Art What Powder Dissolves In Water Water molecules in front of and behind the ions are not shown. water typically dissolves most ionic compounds and polar molecules. As potassium chloride (kcl) dissolves in water, the ions are hydrated. Into a solvent, it is. Nonpolar molecules, such as those found in grease. water and other polar molecules are attracted to ions, as shown in figure. What Powder Dissolves In Water.
From shaunmwilliams.com
Chapter 11 Presentation What Powder Dissolves In Water why do some material disappear in water? a solution is made when a. Into a solvent, it is. Water molecules in front of and behind the ions are not shown. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules, such. What Powder Dissolves In Water.
From publicaffairsworld.com
what dissolves in water science project What Powder Dissolves In Water why do some material disappear in water? The polar water molecules are attracted by the charges on the k + and cl − ions. Nonpolar molecules, such as those found in grease. Into a solvent, it is. Water molecules in front of and behind the ions are not shown. Learn more about how materials react to water in this. What Powder Dissolves In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID What Powder Dissolves In Water Nonpolar molecules, such as those found in grease. The polar water molecules are attracted by the charges on the k + and cl − ions. Learn more about how materials react to water in this bbc bitesize ks1 science. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). water typically dissolves most ionic. What Powder Dissolves In Water.
From www.chemicals.co.uk
What is Solubility? The Chemistry Blog What Powder Dissolves In Water when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Into a solvent, it is. water typically dissolves most ionic compounds and polar molecules. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). Nonpolar molecules, such as those found in grease.. What Powder Dissolves In Water.
From fineartamerica.com
Dissolving Copper Sulphate Crystals Photograph by Andrew Lambert What Powder Dissolves In Water Into a solvent, it is. water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules, such as those found in grease. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). Learn more about how materials react to water in this bbc bitesize ks1 science. a solution is made when a. . What Powder Dissolves In Water.
From www.science-sparks.com
Fun preschool science Making Mixtures What Powder Dissolves In Water Learn more about how materials react to water in this bbc bitesize ks1 science. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. As potassium chloride (kcl) dissolves in water, the ions are hydrated. water and other polar molecules are attracted to ions, as shown in. What Powder Dissolves In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids What Powder Dissolves In Water Water molecules in front of and behind the ions are not shown. As potassium chloride (kcl) dissolves in water, the ions are hydrated. Nonpolar molecules, such as those found in grease. Learn more about how materials react to water in this bbc bitesize ks1 science. Into a solvent, it is. water and other polar molecules are attracted to ions,. What Powder Dissolves In Water.
From wincellulose.com
How to dissolve HPMC in water Shangdun Cellulose What Powder Dissolves In Water Nonpolar molecules, such as those found in grease. The polar water molecules are attracted by the charges on the k + and cl − ions. Learn more about how materials react to water in this bbc bitesize ks1 science. why do some material disappear in water? when you dissolve something in water, like salt or sugar, the water. What Powder Dissolves In Water.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3333021 What Powder Dissolves In Water Into a solvent, it is. water typically dissolves most ionic compounds and polar molecules. As potassium chloride (kcl) dissolves in water, the ions are hydrated. a solution is made when a. Water molecules in front of and behind the ions are not shown. when you dissolve something in water, like salt or sugar, the water molecules surround. What Powder Dissolves In Water.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? in What Powder Dissolves In Water water typically dissolves most ionic compounds and polar molecules. why do some material disappear in water? water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. a solution is made. What Powder Dissolves In Water.
From handsonaswegrow.com
Learn What Dissolves in Water with a Preschool Science Experiment What Powder Dissolves In Water when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Water molecules in front of and behind the ions are not shown. Nonpolar molecules, such as those found in grease. why do some material disappear in water? water and other polar molecules are attracted to ions,. What Powder Dissolves In Water.
From biomonguer.blogspot.com
La matèria... Tipus de matèria What Powder Dissolves In Water water typically dissolves most ionic compounds and polar molecules. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Into a solvent, it is. a solution is made when a. Nonpolar molecules, such as those found in grease. water and other polar molecules are attracted. What Powder Dissolves In Water.
From www.twinkl.kr
What is Dissolving? Answered Twinkl Teaching Wiki What Powder Dissolves In Water Water molecules in front of and behind the ions are not shown. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Nonpolar molecules, such as those found in grease. why do some material disappear in water? As potassium chloride (kcl) dissolves in water, the ions are. What Powder Dissolves In Water.
From fyohytssx.blob.core.windows.net
Sodium Hydroxide In Liquid Form at John Lucas blog What Powder Dissolves In Water water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). Nonpolar molecules, such as those found in grease. As potassium chloride (kcl) dissolves in water, the ions are hydrated. Learn more about how materials react to water in this bbc bitesize ks1 science. a solution is made when a. The polar water molecules are. What Powder Dissolves In Water.
From courses.lumenlearning.com
The Dissolution Process Chemistry What Powder Dissolves In Water Into a solvent, it is. why do some material disappear in water? water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). Learn more about how materials react to water in this bbc bitesize ks1 science. Water molecules in front of and behind the ions are not shown. Nonpolar molecules, such as those found. What Powder Dissolves In Water.
From fphoto.photoshelter.com
science chemistry precipitation reaction nickel chloride Fundamental What Powder Dissolves In Water As potassium chloride (kcl) dissolves in water, the ions are hydrated. The polar water molecules are attracted by the charges on the k + and cl − ions. a solution is made when a. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Into a solvent,. What Powder Dissolves In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids What Powder Dissolves In Water Water molecules in front of and behind the ions are not shown. As potassium chloride (kcl) dissolves in water, the ions are hydrated. Into a solvent, it is. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). when you dissolve something in water, like salt or sugar, the water molecules surround the particles. What Powder Dissolves In Water.
From www.scienceabc.com
Why Does Sugar Disappear When It Dissolves In Water? » ScienceABC What Powder Dissolves In Water Learn more about how materials react to water in this bbc bitesize ks1 science. why do some material disappear in water? Nonpolar molecules, such as those found in grease. Water molecules in front of and behind the ions are not shown. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of. What Powder Dissolves In Water.
From handsonaswegrow.com
Learn What Dissolves in Water with a Preschool Science Experiment What Powder Dissolves In Water The polar water molecules are attracted by the charges on the k + and cl − ions. Into a solvent, it is. Learn more about how materials react to water in this bbc bitesize ks1 science. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. water. What Powder Dissolves In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The What Powder Dissolves In Water when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Into a solvent, it is. Nonpolar molecules, such as those found in grease. As potassium chloride (kcl) dissolves in water, the ions are hydrated. a solution is made when a. water and other polar molecules are. What Powder Dissolves In Water.
From www.thoughtco.com
Dissolving Sugar in Water Chemical or Physical Change? What Powder Dissolves In Water Learn more about how materials react to water in this bbc bitesize ks1 science. water typically dissolves most ionic compounds and polar molecules. a solution is made when a. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. water and other polar molecules are. What Powder Dissolves In Water.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3188660 What Powder Dissolves In Water water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). The polar water molecules are attracted by the charges on the k + and cl − ions. Nonpolar molecules, such as those found in grease. Water molecules in front of and behind the ions are not shown. As potassium chloride (kcl) dissolves in water, the. What Powder Dissolves In Water.
From littlebinsforlittlehands.com
What Dissolves In Water Experiment Little Bins for Little Hands What Powder Dissolves In Water water typically dissolves most ionic compounds and polar molecules. Into a solvent, it is. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). The polar water molecules are attracted by the charges on the k + and cl − ions. Water molecules in front of and behind the ions are not shown. Nonpolar. What Powder Dissolves In Water.
From teachbesideme.com
Dissolving Science Experiment What Dissolves in Water? Teach Beside Me What Powder Dissolves In Water Into a solvent, it is. Water molecules in front of and behind the ions are not shown. As potassium chloride (kcl) dissolves in water, the ions are hydrated. water typically dissolves most ionic compounds and polar molecules. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). The polar water molecules are attracted by. What Powder Dissolves In Water.
From fphoto.photoshelter.com
science chemistry compound sulfur Fundamental Photographs The Art What Powder Dissolves In Water As potassium chloride (kcl) dissolves in water, the ions are hydrated. The polar water molecules are attracted by the charges on the k + and cl − ions. Water molecules in front of and behind the ions are not shown. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). Nonpolar molecules, such as those. What Powder Dissolves In Water.
From www.youtube.com
Powder dissolve in water reverse YouTube What Powder Dissolves In Water a solution is made when a. Nonpolar molecules, such as those found in grease. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). Water molecules in front of and behind the ions are not shown. As potassium chloride (kcl) dissolves in water, the ions are hydrated. why do some material disappear in. What Powder Dissolves In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids What Powder Dissolves In Water water typically dissolves most ionic compounds and polar molecules. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). Learn more about how materials react to water in this bbc bitesize ks1 science. As potassium chloride (kcl) dissolves in water, the ions are hydrated. Water molecules in front of and behind the ions are. What Powder Dissolves In Water.
From www.youtube.com
is talc powder dissolve in water ? 🤔 YouTube What Powder Dissolves In Water why do some material disappear in water? The polar water molecules are attracted by the charges on the k + and cl − ions. water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules, such as those found in grease. a solution is made when a. Learn more about how materials react to water in this. What Powder Dissolves In Water.
From teachbesideme.com
Dissolving Science Experiment What Dissolves in Water? Teach Beside Me What Powder Dissolves In Water a solution is made when a. water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules, such as those found in grease. when you dissolve something in water, like salt or sugar, the water molecules surround the particles of the substance you’re dissolving. Learn more about how materials react to water in this bbc bitesize ks1. What Powder Dissolves In Water.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize What Powder Dissolves In Water a solution is made when a. Water molecules in front of and behind the ions are not shown. water and other polar molecules are attracted to ions, as shown in figure \(\pageindex{2}\). Learn more about how materials react to water in this bbc bitesize ks1 science. Nonpolar molecules, such as those found in grease. As potassium chloride (kcl). What Powder Dissolves In Water.