Equivalence Point Acid Base Titration Definition . Sorting out some confusing terms. In this section we will learn how to. In chemistry, an equivalence point is a term that is used while performing titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The goal is to reach.
from general.chemistrysteps.com
In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. In this section we will learn how to. The goal is to reach. Sorting out some confusing terms. In chemistry, an equivalence point is a term that is used while performing titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been.
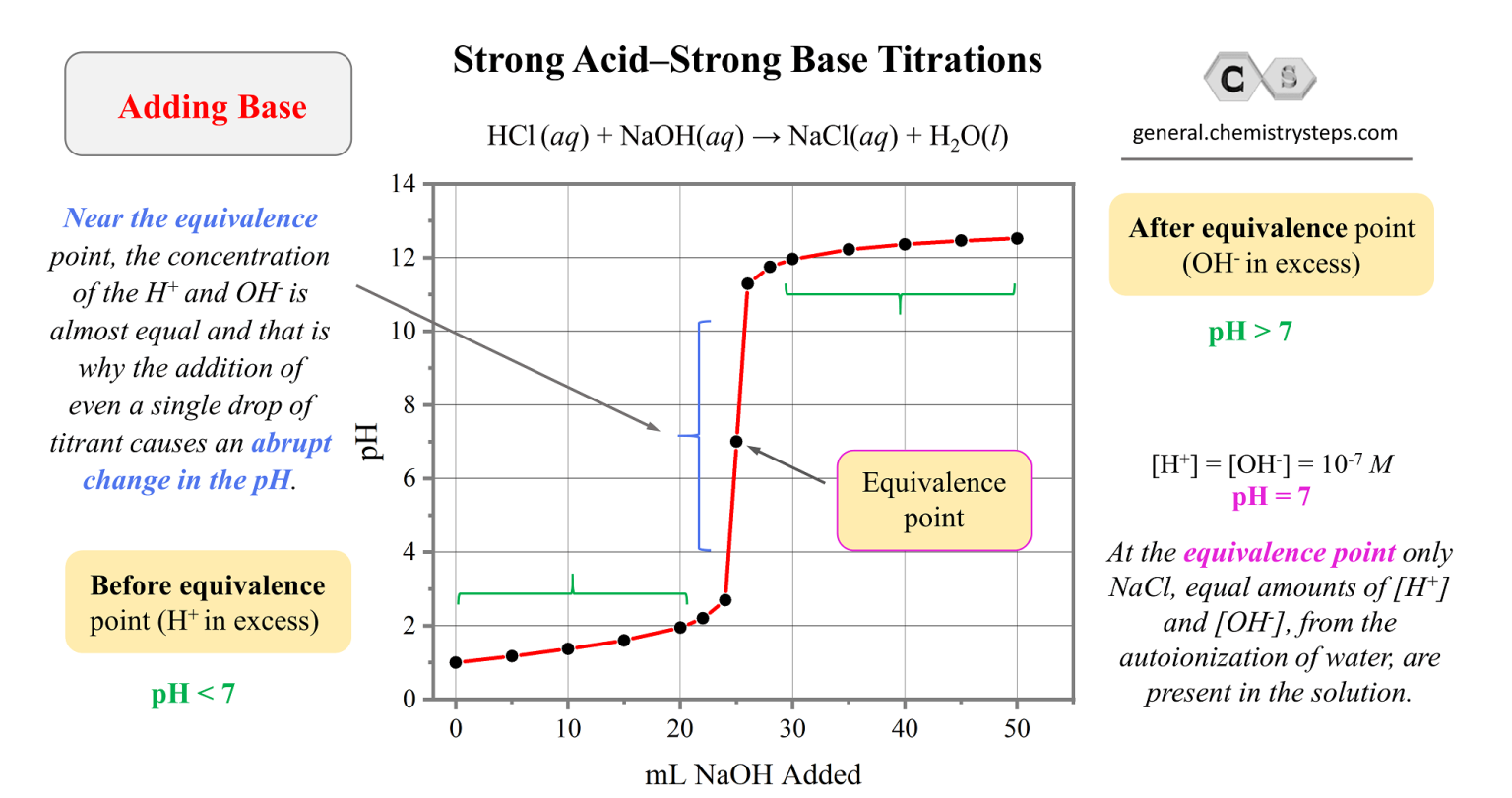
Strong AcidStrong Base Titrations Chemistry Steps
Equivalence Point Acid Base Titration Definition Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. In chemistry, an equivalence point is a term that is used while performing titration. The goal is to reach. In this section we will learn how to. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Equivalence Point Acid Base Titration Definition The goal is to reach. In this section we will learn how to. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. Sorting out some confusing terms. In chemistry, an equivalence point is a term that is used while performing titration. The equivalence point of a titration.. Equivalence Point Acid Base Titration Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Equivalence Point Acid Base Titration Definition In this section we will learn how to. The equivalence point of a titration. In chemistry, an equivalence point is a term that is used while performing titration. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point, or stoichiometric point, of a chemical reaction is the point. Equivalence Point Acid Base Titration Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Equivalence Point Acid Base Titration Definition In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The goal is to reach. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point, or stoichiometric point, of a chemical reaction. Equivalence Point Acid Base Titration Definition.
From www.vrogue.co
How To Calculate Pka From The Half Equivalence Point vrogue.co Equivalence Point Acid Base Titration Definition In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. The goal is to reach. The equivalence point of a. Equivalence Point Acid Base Titration Definition.
From mavink.com
Acid Base Titration Curve Equivalence Point Acid Base Titration Definition In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Sorting out some confusing terms. The goal is to reach. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In this section we will. Equivalence Point Acid Base Titration Definition.
From marielanewslee.blogspot.com
Explain the Difference Between End Point and Equivalence Point Equivalence Point Acid Base Titration Definition In this section we will learn how to. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. Sorting out some confusing terms. The goal is to reach. In the overview to this chapter we noted that a. Equivalence Point Acid Base Titration Definition.
From www.pinterest.ph
Equivalence Point definition The point in a chemical reaction at which Equivalence Point Acid Base Titration Definition In chemistry, an equivalence point is a term that is used while performing titration. In this section we will learn how to. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point of a titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point. Equivalence Point Acid Base Titration Definition.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Equivalence Point Acid Base Titration Definition Sorting out some confusing terms. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In chemistry, an equivalence point is a term that is. Equivalence Point Acid Base Titration Definition.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Equivalence Point Acid Base Titration Definition Sorting out some confusing terms. In this section we will learn how to. The equivalence point of a titration. The goal is to reach. In chemistry, an equivalence point is a term that is used while performing titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been.. Equivalence Point Acid Base Titration Definition.
From www.geeksforgeeks.org
AcidBase Titration Definition, Examples, Types, Key Terms Equivalence Point Acid Base Titration Definition The equivalence point of a titration. The goal is to reach. In this section we will learn how to. In chemistry, an equivalence point is a term that is used while performing titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. Sketch out a plot representing. Equivalence Point Acid Base Titration Definition.
From www.slideshare.net
pH Understanding titration curve Equivalence Point Acid Base Titration Definition Sorting out some confusing terms. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. The equivalence point of a titration. In chemistry, an equivalence point is a term that is used while performing titration. In the overview to this chapter we noted that a titration’s end point. Equivalence Point Acid Base Titration Definition.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Equivalence Point Acid Base Titration Definition The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. The equivalence point of a titration. The goal is to reach. Sorting out some confusing terms. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated. Equivalence Point Acid Base Titration Definition.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Equivalence Point Acid Base Titration Definition The equivalence point of a titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. In chemistry, an equivalence point is a term that is used while performing titration. Sorting out some confusing terms. In the overview to this chapter we noted that a titration’s end point. Equivalence Point Acid Base Titration Definition.
From schoolbag.info
If the acetic acid being titrated here were replaced by hydrochloric Equivalence Point Acid Base Titration Definition In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. In this section we will learn how to. Sorting. Equivalence Point Acid Base Titration Definition.
From www.youtube.com
Acid Base Titration Curves Simplified YouTube Equivalence Point Acid Base Titration Definition Sorting out some confusing terms. In chemistry, an equivalence point is a term that is used while performing titration. The goal is to reach. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. The equivalence point, or. Equivalence Point Acid Base Titration Definition.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Equivalence Point Acid Base Titration Definition In chemistry, an equivalence point is a term that is used while performing titration. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The goal is to reach. In the overview to this chapter we noted that. Equivalence Point Acid Base Titration Definition.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Equivalence Point Acid Base Titration Definition The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. In chemistry, an equivalence point is a term that is used while performing titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. Equivalence Point Acid Base Titration Definition.
From www.youtube.com
What is Equivalence Point (acidbase titrations)? YouTube Equivalence Point Acid Base Titration Definition In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In chemistry, an equivalence point is a term that. Equivalence Point Acid Base Titration Definition.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Equivalence Point Acid Base Titration Definition In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The goal is to reach. The equivalence point of a titration. In this section we will learn how to. In chemistry, an equivalence point is a term that is used while performing titration. Sketch out a plot representing the titration of. Equivalence Point Acid Base Titration Definition.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID9681406 Equivalence Point Acid Base Titration Definition In this section we will learn how to. The goal is to reach. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. In chemistry, an equivalence point is a term that is used while performing titration. The equivalence. Equivalence Point Acid Base Titration Definition.
From www.tes.com
Acid Base Titrations, Neutralization, Equivalence Point Grade 11 Equivalence Point Acid Base Titration Definition In chemistry, an equivalence point is a term that is used while performing titration. The goal is to reach. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. In this section we will learn how to. Sorting out some confusing terms. The equivalence point of a titration. The equivalence point,. Equivalence Point Acid Base Titration Definition.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Equivalence Point Acid Base Titration Definition Sorting out some confusing terms. The equivalence point of a titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. In this section we will learn how to. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point.. Equivalence Point Acid Base Titration Definition.
From www.chegg.com
Solved 1. What is the definition of an 'equivalence point' Equivalence Point Acid Base Titration Definition The equivalence point of a titration. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. The goal is to reach. Sorting out some confusing terms. Sketch out a. Equivalence Point Acid Base Titration Definition.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 Equivalence Point Acid Base Titration Definition In chemistry, an equivalence point is a term that is used while performing titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. The equivalence point of a titration. The goal is to reach. In the overview to this chapter we noted that a titration’s end point. Equivalence Point Acid Base Titration Definition.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Equivalence Point Acid Base Titration Definition In chemistry, an equivalence point is a term that is used while performing titration. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point of a titration. Sorting out some confusing terms. The goal is to reach. Sketch out a plot representing the titration of a weak monoprotic. Equivalence Point Acid Base Titration Definition.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Equivalence Point Acid Base Titration Definition Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. In chemistry, an equivalence point is a. Equivalence Point Acid Base Titration Definition.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Equivalence Point Acid Base Titration Definition In this section we will learn how to. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants. Equivalence Point Acid Base Titration Definition.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Equivalence Point Acid Base Titration Definition The equivalence point of a titration. In chemistry, an equivalence point is a term that is used while performing titration. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have. Equivalence Point Acid Base Titration Definition.
From philschatz.com
AcidBase Titrations · Chemistry Equivalence Point Acid Base Titration Definition Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The goal is to reach. In chemistry, an equivalence point is a term that is. Equivalence Point Acid Base Titration Definition.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Equivalence Point Acid Base Titration Definition Sorting out some confusing terms. In this section we will learn how to. The equivalence point of a titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point.. Equivalence Point Acid Base Titration Definition.
From www.youtube.com
Titrations and the M1V1=M2V2 Math at The Equivalence Point YouTube Equivalence Point Acid Base Titration Definition The equivalence point of a titration. In chemistry, an equivalence point is a term that is used while performing titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. Sorting out some confusing terms. In this section we will learn how to. In the overview to this. Equivalence Point Acid Base Titration Definition.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Equivalence Point Acid Base Titration Definition Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. Sorting out some confusing terms. The. Equivalence Point Acid Base Titration Definition.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Equivalence Point Acid Base Titration Definition In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point of a titration. The goal is to reach. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing. Equivalence Point Acid Base Titration Definition.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Equivalence Point Acid Base Titration Definition The equivalence point of a titration. The goal is to reach. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In this section we will learn how to. Sorting out some confusing terms. In chemistry, an equivalence point is a term that is. Equivalence Point Acid Base Titration Definition.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Equivalence Point Acid Base Titration Definition In chemistry, an equivalence point is a term that is used while performing titration. The goal is to reach. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In the overview to this chapter we noted that a titration’s end point should coincide. Equivalence Point Acid Base Titration Definition.