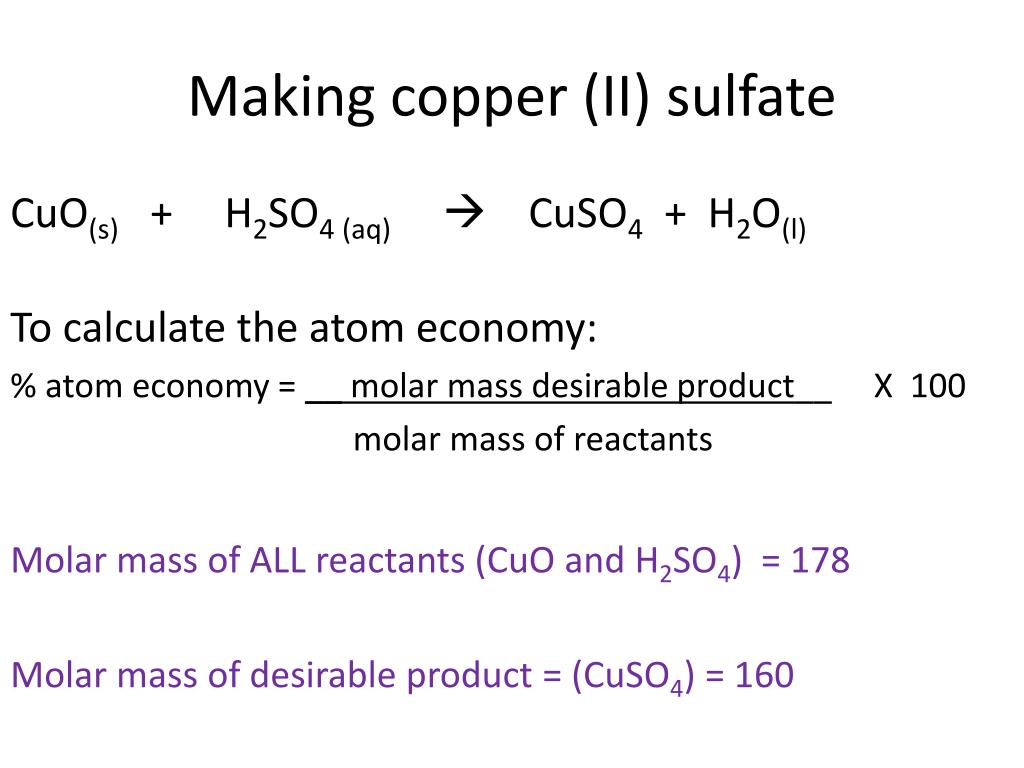
Copper Ii Sulfate Water Balanced Equation . students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. Condensing the vapour produced in a second test tube collects the water. the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t. One mole of copper [cu] and two moles of sulfuric acid [h. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. This is a class experiment suitable for students who already have. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. And so, we write the first part of the.
from exotjinqn.blob.core.windows.net
And so, we write the first part of the. the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. This is a class experiment suitable for students who already have. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. Condensing the vapour produced in a second test tube collects the water.
Copper Ii Sulfate Chemical Formula at Jack Butler blog
Copper Ii Sulfate Water Balanced Equation the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t. the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t. Condensing the vapour produced in a second test tube collects the water. This is a class experiment suitable for students who already have. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. One mole of copper [cu] and two moles of sulfuric acid [h. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. And so, we write the first part of the. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water.
From www.numerade.com
SOLVED Write the balanced chemical equation, including the states, for Copper Ii Sulfate Water Balanced Equation Condensing the vapour produced in a second test tube collects the water. This is a class experiment suitable for students who already have. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. And so, we write the first part of the. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. 1. Copper Ii Sulfate Water Balanced Equation.
From www.tessshebaylo.com
Write A Balanced Chemical Equation For The Of Copper Ii Copper Ii Sulfate Water Balanced Equation And so, we write the first part of the. This is a class experiment suitable for students who already have. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. One mole of. Copper Ii Sulfate Water Balanced Equation.
From www.meritnation.com
balance and write symbol of copper+sulphuric acid gives copper(2 Copper Ii Sulfate Water Balanced Equation in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. Condensing the vapour produced in a second test tube collects the water. the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t. And so, we write the first part of the. students remove the water of. Copper Ii Sulfate Water Balanced Equation.
From chemicaldb.netlify.app
Copper 2 sulfate with water heat Copper Ii Sulfate Water Balanced Equation in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. And so, we write the first part of the. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. Condensing the vapour produced in a second test tube collects the water. 1 copper(ii) sulfate. Copper Ii Sulfate Water Balanced Equation.
From www.toppr.com
1. Write the formula and then balance the following equation. Copper Copper Ii Sulfate Water Balanced Equation we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. Condensing the vapour produced in a second test tube collects the water. the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t.. Copper Ii Sulfate Water Balanced Equation.
From www.youtube.com
Finding the Empirical Formula of Hydrated Copper (II) Sulfate (Updated Copper Ii Sulfate Water Balanced Equation And so, we write the first part of the. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. This is a class experiment suitable for students who already have. Condensing the vapour produced in a second test tube collects the water. in this experiment, a known mass of hydrated copper (ii) sulfate is heated. Copper Ii Sulfate Water Balanced Equation.
From exotjinqn.blob.core.windows.net
Copper Ii Sulfate Chemical Formula at Jack Butler blog Copper Ii Sulfate Water Balanced Equation And so, we write the first part of the. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. Condensing the vapour produced in a second test tube collects the water. 1 copper(ii) sulfate solution, cuso 4. Copper Ii Sulfate Water Balanced Equation.
From dxojfqcbd.blob.core.windows.net
Copper Ii Oxide To Copper Ii Sulfate Equation at Gloria Schirmer blog Copper Ii Sulfate Water Balanced Equation Condensing the vapour produced in a second test tube collects the water. the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. This is a class experiment suitable for students who already have. in this experiment, a known mass of hydrated copper (ii) sulfate. Copper Ii Sulfate Water Balanced Equation.
From drqmlwefeco.blob.core.windows.net
Copper Ii Hydroxide And Ammonia at Lawrence Klein blog Copper Ii Sulfate Water Balanced Equation students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. One mole of copper [cu] and two moles of sulfuric acid [h. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. And so, we write the first part of the. Copper + sulfuric acid = cupric sulfate + sulfur dioxide. Copper Ii Sulfate Water Balanced Equation.
From studylib.net
To find the formula of hydrated copper(II) sulfate Copper Ii Sulfate Water Balanced Equation the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. Condensing the vapour produced in a second test tube collects the water. This is a class experiment suitable for students who already have. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by. Copper Ii Sulfate Water Balanced Equation.
From www.tessshebaylo.com
Convert Words To Chemical Equations Tessshebaylo Copper Ii Sulfate Water Balanced Equation This is a class experiment suitable for students who already have. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. And so, we write the first part of the. Condensing the vapour produced in a second test tube collects the water. write a balanced equation for the decomposition. Copper Ii Sulfate Water Balanced Equation.
From www.numerade.com
SOLVED Tetraamminecopper(II) Sulfate The first step in the synthesis Copper Ii Sulfate Water Balanced Equation Condensing the vapour produced in a second test tube collects the water. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. And so, we write the first part of the. This is a class experiment suitable for students who already have. Copper + sulfuric acid = cupric sulfate +. Copper Ii Sulfate Water Balanced Equation.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Copper Ii Sulfate Water Balanced Equation 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. Condensing the vapour produced in a second test tube collects the water. One mole of copper [cu] and two moles. Copper Ii Sulfate Water Balanced Equation.
From www.vrogue.co
How To Write The Formula For Copper Ii Sulfate Youtub vrogue.co Copper Ii Sulfate Water Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. This is a class experiment suitable for students who already have. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made. Copper Ii Sulfate Water Balanced Equation.
From brainly.com
24) Write, balance, and label the equations below. HCl(aq) + KOH(aq Copper Ii Sulfate Water Balanced Equation This is a class experiment suitable for students who already have. Condensing the vapour produced in a second test tube collects the water. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. students remove the water of crystallisation. Copper Ii Sulfate Water Balanced Equation.
From www.ashinthewild.com
Iron And Copper Sulfate Balanced Equation Ash in The Wild Copper Ii Sulfate Water Balanced Equation students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. Condensing the vapour produced in a second test tube collects the water. we start off with the anhydrous copper(ii) sulfate crystal, which is white in. Copper Ii Sulfate Water Balanced Equation.
From www.toppr.com
Write a balanced equation for the preparation of the following salts.(i Copper Ii Sulfate Water Balanced Equation And so, we write the first part of the. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. One mole of copper [cu] and two moles of sulfuric acid [h. Condensing the. Copper Ii Sulfate Water Balanced Equation.
From edu.rsc.org
Finding the formula of hydrated copper(II) sulfate Experiment RSC Copper Ii Sulfate Water Balanced Equation 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. One mole of copper [cu] and two moles of sulfuric acid [h. write a balanced equation for the decomposition. Copper Ii Sulfate Water Balanced Equation.
From liamatmaldonado.blogspot.com
Copper Ii Sulfate Pentahydrate Dissolved Water Chemical Equation Copper Ii Sulfate Water Balanced Equation the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. Condensing the vapour produced in a second test tube collects the water. And so, we write the first part of. Copper Ii Sulfate Water Balanced Equation.
From www.youtube.com
How to Balance Copper (II) sulfate + Ammonium hydroxide YouTube Copper Ii Sulfate Water Balanced Equation we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. This is a class experiment suitable for students who already have. One mole of copper [cu] and two moles of sulfuric acid [h. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. students remove the water of crystallisation from hydrated copper. Copper Ii Sulfate Water Balanced Equation.
From cristianmeowterry.blogspot.com
Molecular Equation for Copper Ii Sulfate and Sodium Phosphate Copper Ii Sulfate Water Balanced Equation One mole of copper [cu] and two moles of sulfuric acid [h. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. write a balanced equation for the decomposition of ammonium nitrate. Copper Ii Sulfate Water Balanced Equation.
From slideplayer.com
Chapter Thirteen CHEMICAL EQUILIBRIUM. ppt download Copper Ii Sulfate Water Balanced Equation And so, we write the first part of the. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. Condensing the vapour produced in a second test tube collects the water. 1 copper(ii) sulfate solution, cuso 4 (aq), can be. Copper Ii Sulfate Water Balanced Equation.
From dxojfqcbd.blob.core.windows.net
Copper Ii Oxide To Copper Ii Sulfate Equation at Gloria Schirmer blog Copper Ii Sulfate Water Balanced Equation write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. And so, we write the first part of the. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. . Copper Ii Sulfate Water Balanced Equation.
From exospwven.blob.core.windows.net
Copper (Ii) Sulfate Is The And Water Is The at Ben Pratt blog Copper Ii Sulfate Water Balanced Equation Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. One mole of copper [cu] and two moles of sulfuric acid [h. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. . Copper Ii Sulfate Water Balanced Equation.
From childhealthpolicy.vumc.org
🌷 Hydrated copper sulfate equation. Finding the formula of hydrated Copper Ii Sulfate Water Balanced Equation Condensing the vapour produced in a second test tube collects the water. And so, we write the first part of the. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. we start off with the anhydrous copper(ii) sulfate. Copper Ii Sulfate Water Balanced Equation.
From www.gbu-presnenskij.ru
Equation For CuSO4 H2O Copper (II) Sulfate Water, 40 OFF Copper Ii Sulfate Water Balanced Equation in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. And so, we write the first part of the. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular. Copper Ii Sulfate Water Balanced Equation.
From www.numerade.com
SOLVED When sulfuric acid and copper (II) oxide are allowed to react Copper Ii Sulfate Water Balanced Equation we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. This is a class experiment suitable for students who already have. write a balanced equation for the decomposition of ammonium nitrate to form molecular. Copper Ii Sulfate Water Balanced Equation.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write Copper Ii Sulfate Water Balanced Equation students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. Condensing the vapour produced in a second test tube collects the water. we start off with the anhydrous copper(ii) sulfate. Copper Ii Sulfate Water Balanced Equation.
From www.toppr.com
Write balanced chemical equations the following word equationCopper Copper Ii Sulfate Water Balanced Equation students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. Condensing the vapour produced in a second test tube collects the water. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. we. Copper Ii Sulfate Water Balanced Equation.
From www.coursehero.com
[Solved] iron filings added to copper(II) sulfate in solution (assume Copper Ii Sulfate Water Balanced Equation This is a class experiment suitable for students who already have. the equation for cuso4 ( copper (ii) sulfate and h2o sometimes isn’t. One mole of copper [cu] and two moles of sulfuric acid [h. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. in this experiment, a known mass of hydrated copper. Copper Ii Sulfate Water Balanced Equation.
From www.youtube.com
How to separate sand and copper(II) sulfate salt from its mixture Copper Ii Sulfate Water Balanced Equation in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. And so, we write the first part of the. One mole of copper [cu] and two moles of sulfuric acid [h. we start off with the anhydrous copper(ii) sulfate crystal, which is white in colour. write a balanced. Copper Ii Sulfate Water Balanced Equation.
From cesoxxly.blob.core.windows.net
Copper 2 Sulfate Dissociation Equation at David Robison blog Copper Ii Sulfate Water Balanced Equation Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. in this experiment, a known mass of hydrated copper (ii) sulfate is heated to remove the water of crystallisation. Condensing the vapour produced in a second test tube collects the water. This is a class experiment suitable for students who already have. the equation for cuso4. Copper Ii Sulfate Water Balanced Equation.
From www.coursehero.com
[Solved] copper(II)sulfate + Barium chloride molecular equation and Copper Ii Sulfate Water Balanced Equation 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Copper + sulfuric. Copper Ii Sulfate Water Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the singlereplacement oxidation Copper Ii Sulfate Water Balanced Equation One mole of copper [cu] and two moles of sulfuric acid [h. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. And so, we write the first part of the.. Copper Ii Sulfate Water Balanced Equation.
From www.youtube.com
DEMONSTRATING USING COPPER(II) SULFATE TO DETECT WATER A REVERSIBLE Copper Ii Sulfate Water Balanced Equation Copper + sulfuric acid = cupric sulfate + sulfur dioxide + water. students remove the water of crystallisation from hydrated copper (ii) sulfate by heating. 1 copper(ii) sulfate solution, cuso 4 (aq), can be made by adding an excess of solid copper(ii) oxide, cuo, to boiling dilute sulfuric acid. we start off with the anhydrous copper(ii) sulfate. Copper Ii Sulfate Water Balanced Equation.