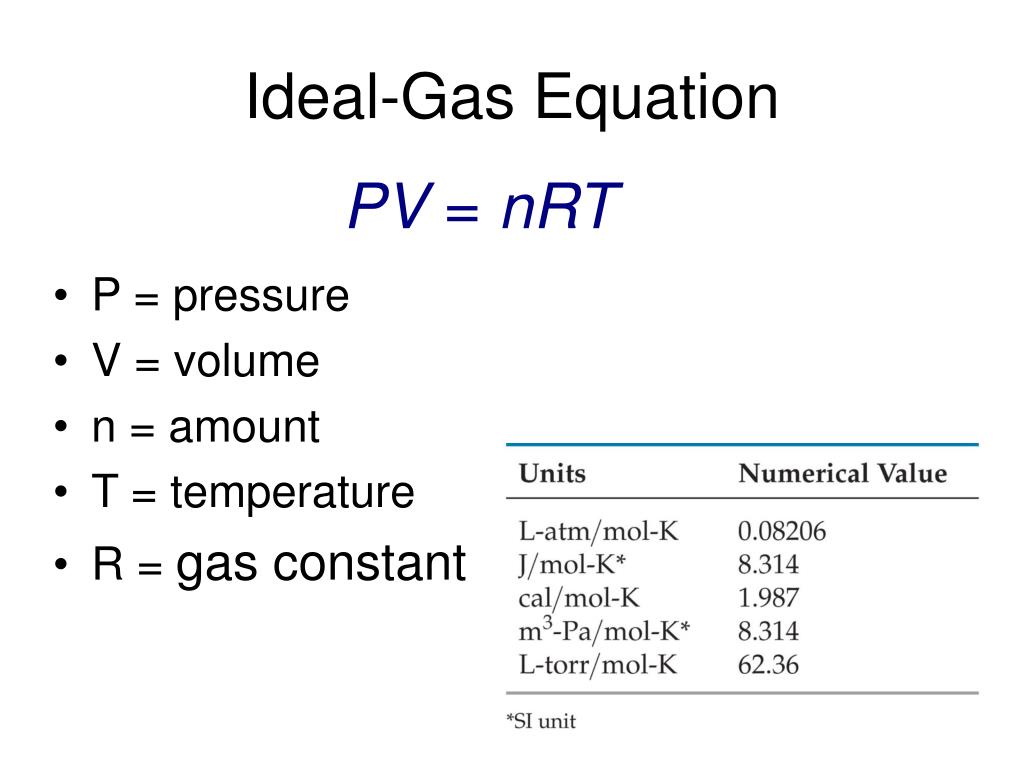
What Is K In Pv=K . Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. boyle's law states that when temperature is constant: V symbolizes the gas’s volume. I.e., pv = k, a. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. The equation for this is pv = k,. P symbolizes the pressure of the gas. Boyle's law also states that pressure is inversely related to volume. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; K remains unchanging as long as temperature and gas quantity remain.
from tasubtitlex.weebly.com
The equation for this is pv = k,. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. P symbolizes the pressure of the gas. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. V symbolizes the gas’s volume. K remains unchanging as long as temperature and gas quantity remain. I.e., pv = k, a. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; boyle's law states that when temperature is constant: Boyle's law also states that pressure is inversely related to volume.
Ideal gas law weather calculator tasubtitleX
What Is K In Pv=K I.e., pv = k, a. Boyle's law also states that pressure is inversely related to volume. P symbolizes the pressure of the gas. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; The equation for this is pv = k,. K remains unchanging as long as temperature and gas quantity remain. V symbolizes the gas’s volume. boyle's law states that when temperature is constant: I.e., pv = k, a. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a.
From studylib.net
PV = k or P1V1 = P2V2 P1V1 = P2V2 P1V1 = P2V2 ( ) What Is K In Pv=K I.e., pv = k, a. boyle's law states that when temperature is constant: the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. The equation for. What Is K In Pv=K.
From brainly.com
According to the Ideal Gas Law,PV = kT,where P is pressure, V is volume What Is K In Pv=K P symbolizes the pressure of the gas. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. boyle's law states that when temperature is constant: K. What Is K In Pv=K.
From chemistryguru.com.sg
Ideal Gas Graph Sketching What Is K In Pv=K P symbolizes the pressure of the gas. The equation for this is pv = k,. V symbolizes the gas’s volume. I.e., pv = k, a. boyle's law states that when temperature is constant: the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; simply. What Is K In Pv=K.
From tasubtitlex.weebly.com
Ideal gas law weather calculator tasubtitleX What Is K In Pv=K Boyle's law also states that pressure is inversely related to volume. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. V symbolizes the gas’s volume. The equation for this is pv = k,. the empirical relation asserts that the pressure (p) of a given quantity of gas changes. What Is K In Pv=K.
From www.slideserve.com
PPT Gases Chapter 10/11 Modern Chemistry PowerPoint Presentation What Is K In Pv=K V symbolizes the gas’s volume. boyle's law states that when temperature is constant: Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. P symbolizes the pressure of the gas.. What Is K In Pv=K.
From www.numerade.com
SOLVED The pressure volume and temperature Of mole of an ideal gas are What Is K In Pv=K boyle's law states that when temperature is constant: V symbolizes the gas’s volume. The equation for this is pv = k,. K remains unchanging as long as temperature and gas quantity remain. P symbolizes the pressure of the gas. I.e., pv = k, a. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. What Is K In Pv=K.
From brainly.in
PV=K we can say that Brainly.in What Is K In Pv=K The equation for this is pv = k,. K remains unchanging as long as temperature and gas quantity remain. P symbolizes the pressure of the gas. Boyle's law also states that pressure is inversely related to volume. boyle's law states that when temperature is constant: Boyle’s law gives the relationship between the pressure of a gas and the volume. What Is K In Pv=K.
From www.numerade.com
SOLVEDThe ?alpor pressures of salid and liquid bydrogen iodide cun be What Is K In Pv=K V symbolizes the gas’s volume. K remains unchanging as long as temperature and gas quantity remain. Boyle's law also states that pressure is inversely related to volume. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. The equation for this is pv = k,. the empirical relation asserts. What Is K In Pv=K.
From chemistryguru.com.sg
2020 P1 Q6 Compare PV vs V Graph of Equal Masses of Gases What Is K In Pv=K boyle's law states that when temperature is constant: I.e., pv = k, a. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. Boyle's law also states that pressure is inversely related to volume. K remains unchanging as long as temperature and gas quantity remain. the empirical relation. What Is K In Pv=K.
From www.youtube.com
Gas Laws Boyle's Law (PV = k) YouTube What Is K In Pv=K K remains unchanging as long as temperature and gas quantity remain. boyle's law states that when temperature is constant: Boyle's law also states that pressure is inversely related to volume. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. P symbolizes the pressure of the gas. I.e., pv. What Is K In Pv=K.
From www.meritnation.com
Explain Boyle's Law and its graphical representation also Chemistry What Is K In Pv=K The equation for this is pv = k,. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; P symbolizes the pressure of the gas. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. V symbolizes. What Is K In Pv=K.
From www.sliderbase.com
The Ideal Gas Law Presentation Chemistry What Is K In Pv=K boyle's law states that when temperature is constant: K remains unchanging as long as temperature and gas quantity remain. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; The equation for this is pv = k,. simply put, boyle's states that for a. What Is K In Pv=K.
From www.slideshare.net
Gas laws Diagrams What Is K In Pv=K K remains unchanging as long as temperature and gas quantity remain. V symbolizes the gas’s volume. boyle's law states that when temperature is constant: Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. I.e., pv = k, a. Boyle's law also states that pressure is inversely related to volume.. What Is K In Pv=K.
From www.chegg.com
Solved PV = MRT Where P is pressure, V is volume, M is What Is K In Pv=K V symbolizes the gas’s volume. The equation for this is pv = k,. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; K remains unchanging as. What Is K In Pv=K.
From www.numerade.com
SOLVED Q1. The universal ideal gas equation was derived to work What Is K In Pv=K K remains unchanging as long as temperature and gas quantity remain. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; boyle's law states that when temperature is constant: Boyle's law also states that pressure is inversely related to volume. Boyle’s law gives the relationship. What Is K In Pv=K.
From www.numerade.com
SOLVED The ideal gas equation is PV = nRT, where P is pressure, V is What Is K In Pv=K the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; K remains unchanging as long as temperature and gas quantity remain. The equation for this is pv = k,. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is. What Is K In Pv=K.
From www.gauthmath.com
Solved d.Boyle's Law PV = k, where P is the pressure an is the volume What Is K In Pv=K I.e., pv = k, a. K remains unchanging as long as temperature and gas quantity remain. V symbolizes the gas’s volume. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; boyle's law states that when temperature is constant: simply put, boyle's states that. What Is K In Pv=K.
From mmerevise.co.uk
The Ideal Gas Equation MME What Is K In Pv=K Boyle's law also states that pressure is inversely related to volume. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. P symbolizes the pressure of the gas. The equation for this is pv = k,. Boyle’s law gives the relationship between the pressure of a gas and the volume. What Is K In Pv=K.
From stock.adobe.com
PV = nRT Ideal Gas Law Brings Together Gas Properties. The Most What Is K In Pv=K the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; boyle's law states that when temperature is constant: Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. I.e., pv = k, a. Boyle's law also. What Is K In Pv=K.
From london-bookschapter.blogspot.com
London Books Chapter What Is K In Pv=K I.e., pv = k, a. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. Boyle's law also states that pressure is inversely related to volume. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. K remains unchanging as long. What Is K In Pv=K.
From studylib.es
PV K What Is K In Pv=K Boyle's law also states that pressure is inversely related to volume. P symbolizes the pressure of the gas. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. V symbolizes the. What Is K In Pv=K.
From studylib.net
BOYLES' LAW PV = k where P = pressure, V = volume, and k = c What Is K In Pv=K the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; The equation for this is pv = k,. I.e., pv = k, a. K remains unchanging as long as temperature and gas quantity remain. boyle's law states that when temperature is constant: simply put,. What Is K In Pv=K.
From slidesharenow.blogspot.com
What Is The N In Pv Nrt slideshare What Is K In Pv=K Boyle's law also states that pressure is inversely related to volume. V symbolizes the gas’s volume. I.e., pv = k, a. The equation for this is pv = k,. boyle's law states that when temperature is constant: P symbolizes the pressure of the gas. the empirical relation asserts that the pressure (p) of a given quantity of gas. What Is K In Pv=K.
From www.youtube.com
งานเนื่องจากการเปลี่ยนแปลงปริมาตรในรูปของ PV Diagram YouTube What Is K In Pv=K V symbolizes the gas’s volume. Boyle's law also states that pressure is inversely related to volume. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. The equation for this is pv = k,. the empirical relation asserts that the pressure (p) of a given quantity of gas changes. What Is K In Pv=K.
From diagramdataadducting.z21.web.core.windows.net
Pv Diagram Of Propane What Is K In Pv=K K remains unchanging as long as temperature and gas quantity remain. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. V symbolizes the gas’s volume. P symbolizes the pressure of the gas. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely. What Is K In Pv=K.
From chem.libretexts.org
Chapter 11.1 Real Gases Chemistry LibreTexts What Is K In Pv=K the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; P symbolizes the pressure of the gas. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. The equation for this is pv = k,. boyle's. What Is K In Pv=K.
From slidesharenow.blogspot.com
What Is The N In Pv Nrt slideshare What Is K In Pv=K The equation for this is pv = k,. boyle's law states that when temperature is constant: Boyle's law also states that pressure is inversely related to volume. V symbolizes the gas’s volume. I.e., pv = k, a. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. K remains. What Is K In Pv=K.
From brainly.in
PV=K we can say that Brainly.in What Is K In Pv=K P symbolizes the pressure of the gas. V symbolizes the gas’s volume. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; K remains unchanging as long as temperature and gas quantity remain. I.e., pv = k, a. Boyle's law also states that pressure is inversely. What Is K In Pv=K.
From quizdborthoclase.z13.web.core.windows.net
What Is A Monatomic Gas What Is K In Pv=K V symbolizes the gas’s volume. I.e., pv = k, a. K remains unchanging as long as temperature and gas quantity remain. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a.. What Is K In Pv=K.
From www.youtube.com
施工動画 PVKスライド[R1タイプ] YouTube What Is K In Pv=K boyle's law states that when temperature is constant: P symbolizes the pressure of the gas. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; The. What Is K In Pv=K.
From www.slideserve.com
PPT P 1 V 1 = P 2 V 2 PowerPoint Presentation, free download ID6032006 What Is K In Pv=K P symbolizes the pressure of the gas. Boyle's law also states that pressure is inversely related to volume. I.e., pv = k, a. V symbolizes the gas’s volume. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. The equation for this is pv = k,. boyle's law states. What Is K In Pv=K.
From www.youtube.com
Thermodynamics Lecture 10 Polytropic Processes YouTube What Is K In Pv=K V symbolizes the gas’s volume. P symbolizes the pressure of the gas. Boyle's law also states that pressure is inversely related to volume. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. K remains unchanging as long as temperature and gas quantity remain. I.e., pv = k, a. simply. What Is K In Pv=K.
From chemistryguru.com.sg
Physical Chemistry Video Lessons What Is K In Pv=K I.e., pv = k, a. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value. Boyle's law also states that pressure is inversely related to volume. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. the empirical relation asserts. What Is K In Pv=K.
From www.eigenplus.com
Polytropic process Equation, Work done Explanation eigenplus What Is K In Pv=K K remains unchanging as long as temperature and gas quantity remain. Boyle’s law gives the relationship between the pressure of a gas and the volume of the gas at a. I.e., pv = k, a. V symbolizes the gas’s volume. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by volume is a constant value.. What Is K In Pv=K.
From www.youtube.com
Gas Laws Combined (PV/T = k) YouTube What Is K In Pv=K the empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; The equation for this is pv = k,. boyle's law states that when temperature is constant: Boyle's law also states that pressure is inversely related to volume. Boyle’s law gives the relationship between the pressure. What Is K In Pv=K.