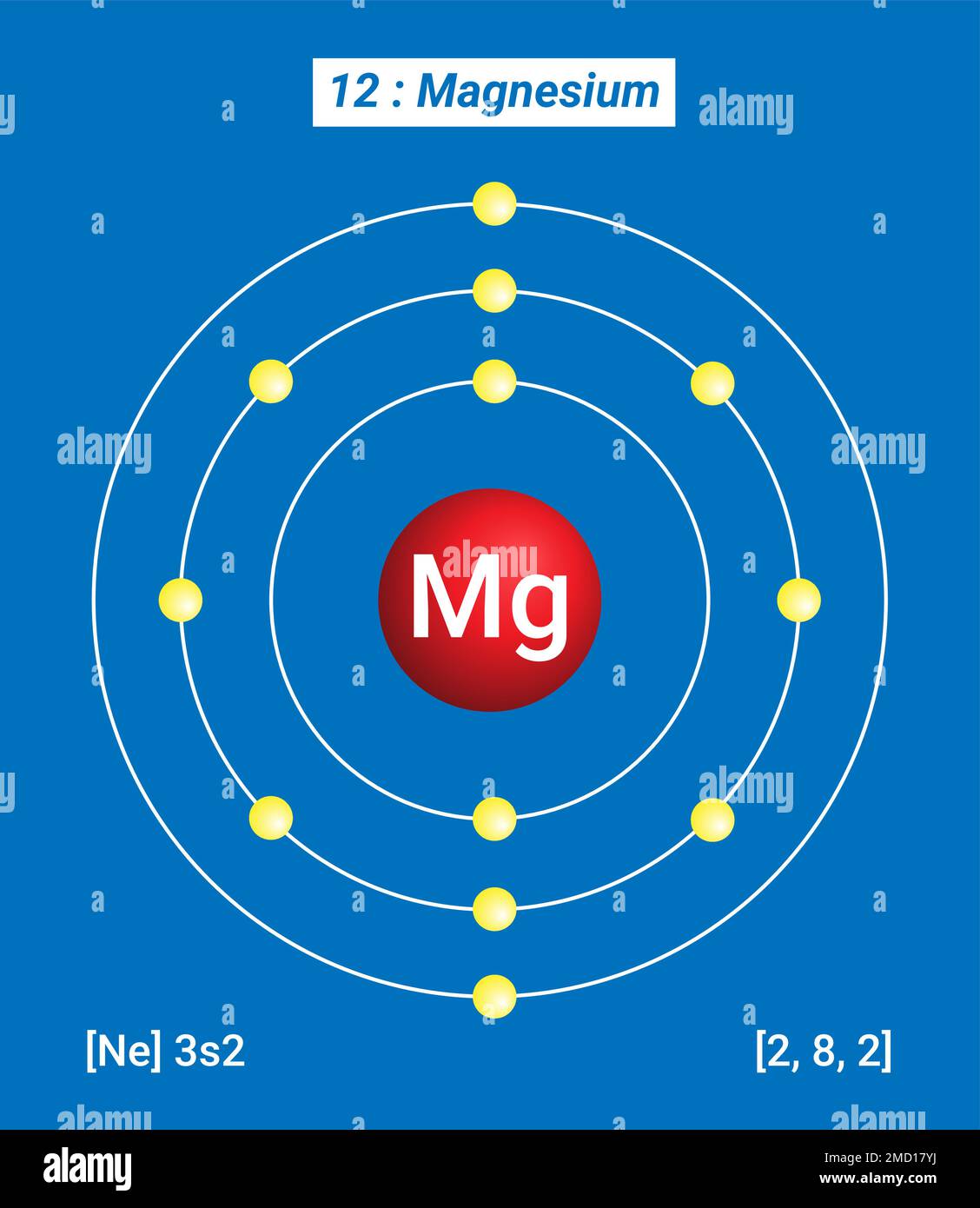
Magnesium Ion Element Compound . That is, does it have ionic bonds, or covalent bonds? The ions play a major role in. Magnesium typically forms ionic bonds, owing to its two valence electrons. Magnesium is a group two element and is the eighth most common element in the earth's crust. Magnesium is the 11th most abundant element by mass in the human body. Sources, facts, uses, scarcity (sri), podcasts, alchemical. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. The first question we ask is if the compound is ionic or covalent? Its compounds are widely used in. Its ions are essential to all living cells.
from www.alamy.com
Magnesium typically forms ionic bonds, owing to its two valence electrons. Magnesium is a group two element and is the eighth most common element in the earth's crust. Its compounds are widely used in. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Its ions are essential to all living cells. That is, does it have ionic bonds, or covalent bonds? A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. The first question we ask is if the compound is ionic or covalent? Magnesium is the 11th most abundant element by mass in the human body. The ions play a major role in.
Mg Magnesium, Periodic Table of the Elements, Shell Structure of
Magnesium Ion Element Compound Magnesium is the 11th most abundant element by mass in the human body. Sources, facts, uses, scarcity (sri), podcasts, alchemical. The first question we ask is if the compound is ionic or covalent? A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium is the 11th most abundant element by mass in the human body. Magnesium typically forms ionic bonds, owing to its two valence electrons. That is, does it have ionic bonds, or covalent bonds? Its ions are essential to all living cells. The ions play a major role in. Its compounds are widely used in. Magnesium is a group two element and is the eighth most common element in the earth's crust.
From www.youtube.com
Magnesium Electron Configuration YouTube Magnesium Ion Element Compound Its ions are essential to all living cells. Magnesium is a group two element and is the eighth most common element in the earth's crust. Its compounds are widely used in. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium is the 11th most abundant element. Magnesium Ion Element Compound.
From stock.adobe.com
Magnesium (Mg) icon structure chemical element round shape circle green Magnesium Ion Element Compound Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium typically forms ionic bonds, owing to its two valence electrons. The first question we ask is if the compound is ionic or covalent? The ions play a major role in. Its ions are essential to all living cells. Magnesium is the 11th most abundant element by mass in the human body. Its. Magnesium Ion Element Compound.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Ion Element Compound The first question we ask is if the compound is ionic or covalent? Its ions are essential to all living cells. Magnesium is a group two element and is the eighth most common element in the earth's crust. Its compounds are widely used in. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium is the 11th most abundant element by mass. Magnesium Ion Element Compound.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Magnesium Ion Element Compound The first question we ask is if the compound is ionic or covalent? Its compounds are widely used in. That is, does it have ionic bonds, or covalent bonds? Magnesium typically forms ionic bonds, owing to its two valence electrons. Magnesium is the 11th most abundant element by mass in the human body. The ions play a major role in.. Magnesium Ion Element Compound.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Element Compound Magnesium is a group two element and is the eighth most common element in the earth's crust. Sources, facts, uses, scarcity (sri), podcasts, alchemical. The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? The ions play a major role in. Magnesium typically forms ionic bonds, owing. Magnesium Ion Element Compound.
From www.alamy.com
Magnesium chemical element, Sign with atomic number and atomic weight Magnesium Ion Element Compound Its compounds are widely used in. Its ions are essential to all living cells. The ions play a major role in. That is, does it have ionic bonds, or covalent bonds? Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium typically forms ionic bonds, owing to its two valence electrons. Magnesium is the 11th most abundant element by mass in the. Magnesium Ion Element Compound.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Ion Element Compound Magnesium is the 11th most abundant element by mass in the human body. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Sources, facts, uses, scarcity (sri), podcasts, alchemical. That is, does it have ionic bonds, or covalent bonds? Magnesium typically forms ionic bonds, owing to its. Magnesium Ion Element Compound.
From www.americanelements.com
Magnesium (Mg) AMERICAN ELEMENTSs Magnesium Ion Element Compound The ions play a major role in. Magnesium typically forms ionic bonds, owing to its two valence electrons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Sources, facts, uses, scarcity (sri), podcasts, alchemical. The first question we ask is if the compound is ionic or covalent?. Magnesium Ion Element Compound.
From www.youtube.com
How to find the Oxidation Number for the Mg2+ ion. (Magnesium ion Magnesium Ion Element Compound The ions play a major role in. Magnesium typically forms ionic bonds, owing to its two valence electrons. Its ions are essential to all living cells. Sources, facts, uses, scarcity (sri), podcasts, alchemical. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. The first question we ask. Magnesium Ion Element Compound.
From galvinconanstuart.blogspot.com
Lewis Dot Diagram For Magnesium General Wiring Diagram Magnesium Ion Element Compound The ions play a major role in. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium typically forms ionic bonds, owing to its two valence electrons. That is, does it have ionic bonds, or covalent bonds? The first question we ask is if the compound is. Magnesium Ion Element Compound.
From medium.com
What is Magnesium? Periodic Table Elements Medium Periodic Table Magnesium Ion Element Compound Magnesium is a group two element and is the eighth most common element in the earth's crust. Sources, facts, uses, scarcity (sri), podcasts, alchemical. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium is the 11th most abundant element by mass in the human body. Magnesium. Magnesium Ion Element Compound.
From www.alamy.com
Mg Magnesium, Periodic Table of the Elements, Shell Structure of Magnesium Ion Element Compound Its compounds are widely used in. Magnesium is the 11th most abundant element by mass in the human body. Magnesium typically forms ionic bonds, owing to its two valence electrons. The ions play a major role in. Its ions are essential to all living cells. A magnesium atom must lose two electrons to have the same number electrons as an. Magnesium Ion Element Compound.
From www.alamy.com
Chemical element magnesium hires stock photography and images Alamy Magnesium Ion Element Compound A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Its ions are essential to all living cells. The ions play a major role in. That is, does it have ionic bonds, or covalent bonds? Magnesium typically forms ionic bonds, owing to its two valence electrons. Its compounds. Magnesium Ion Element Compound.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Element Compound A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Its ions are essential to all living cells. Sources, facts, uses, scarcity (sri), podcasts, alchemical. The ions play a major role in. The first question we ask is if the compound is ionic or covalent? Magnesium typically forms. Magnesium Ion Element Compound.
From www.youtube.com
Is MgF2 (Magnesium fluoride) Ionic or Covalent? YouTube Magnesium Ion Element Compound The ions play a major role in. Its compounds are widely used in. The first question we ask is if the compound is ionic or covalent? Magnesium is a group two element and is the eighth most common element in the earth's crust. Sources, facts, uses, scarcity (sri), podcasts, alchemical. A magnesium atom must lose two electrons to have the. Magnesium Ion Element Compound.
From www.neuroneeds.com
MAGNESIUM Neuroneeds Magnesium Ion Element Compound That is, does it have ionic bonds, or covalent bonds? A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. The ions play a major role in. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium typically forms ionic bonds, owing to its two valence electrons. The first question. Magnesium Ion Element Compound.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion Element Compound The ions play a major role in. Magnesium is the 11th most abundant element by mass in the human body. The first question we ask is if the compound is ionic or covalent? Magnesium is a group two element and is the eighth most common element in the earth's crust. Its compounds are widely used in. Magnesium typically forms ionic. Magnesium Ion Element Compound.
From www.vecteezy.com
Magnesium symbol. Chemical element of the periodic table. Vector Magnesium Ion Element Compound Magnesium typically forms ionic bonds, owing to its two valence electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium is a group two element and is the eighth most common element in the earth's crust. The first question we ask is if the compound is ionic or covalent? Its compounds are widely used in. Magnesium is the 11th most abundant. Magnesium Ion Element Compound.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Ion Element Compound Magnesium is the 11th most abundant element by mass in the human body. The ions play a major role in. That is, does it have ionic bonds, or covalent bonds? Its compounds are widely used in. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium typically. Magnesium Ion Element Compound.
From www.shutterstock.com
Magnesium Symbol Chemical Element Periodic Table Stock Vector (Royalty Magnesium Ion Element Compound Sources, facts, uses, scarcity (sri), podcasts, alchemical. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Its compounds are widely used in. Magnesium is a group two element and is the eighth most common element in the earth's crust. Its ions are essential to all living cells.. Magnesium Ion Element Compound.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Element Compound That is, does it have ionic bonds, or covalent bonds? Magnesium is the 11th most abundant element by mass in the human body. Its compounds are widely used in. Magnesium is a group two element and is the eighth most common element in the earth's crust. Its ions are essential to all living cells. Sources, facts, uses, scarcity (sri), podcasts,. Magnesium Ion Element Compound.
From www.britannica.com
Magnesium Description, Properties, & Compounds Britannica Magnesium Ion Element Compound Magnesium typically forms ionic bonds, owing to its two valence electrons. The first question we ask is if the compound is ionic or covalent? Magnesium is a group two element and is the eighth most common element in the earth's crust. Sources, facts, uses, scarcity (sri), podcasts, alchemical. That is, does it have ionic bonds, or covalent bonds? Magnesium is. Magnesium Ion Element Compound.
From www.alamy.com
Icon of the element magnesium of the periodic table with representation Magnesium Ion Element Compound Its ions are essential to all living cells. Magnesium is the 11th most abundant element by mass in the human body. That is, does it have ionic bonds, or covalent bonds? Sources, facts, uses, scarcity (sri), podcasts, alchemical. The first question we ask is if the compound is ionic or covalent? A magnesium atom must lose two electrons to have. Magnesium Ion Element Compound.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Ion Element Compound Its ions are essential to all living cells. Magnesium is the 11th most abundant element by mass in the human body. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium is a group two element and is the eighth. Magnesium Ion Element Compound.
From www.alamy.com
Chemical element magnesium hires stock photography and images Alamy Magnesium Ion Element Compound Its compounds are widely used in. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. The ions play a major role in. That is, does it have ionic bonds, or covalent bonds? The first question we ask is if the compound is ionic or covalent? Its ions. Magnesium Ion Element Compound.
From www.vectorstock.com
Periodic table element magnesium icon Royalty Free Vector Magnesium Ion Element Compound Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium is a group two element and is the eighth most common element in the earth's crust. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium typically forms ionic bonds, owing to its two valence electrons. The first question. Magnesium Ion Element Compound.
From favpng.com
Ion Magnesium Carbonate Chemical Compound Compound, PNG Magnesium Ion Element Compound Its compounds are widely used in. Its ions are essential to all living cells. Sources, facts, uses, scarcity (sri), podcasts, alchemical. The ions play a major role in. The first question we ask is if the compound is ionic or covalent? Magnesium typically forms ionic bonds, owing to its two valence electrons. Magnesium is the 11th most abundant element by. Magnesium Ion Element Compound.
From www.webelements.com
Elements Periodic Table » Magnesium » properties of free atoms Magnesium Ion Element Compound Magnesium typically forms ionic bonds, owing to its two valence electrons. Its ions are essential to all living cells. Magnesium is the 11th most abundant element by mass in the human body. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. The first question we ask is. Magnesium Ion Element Compound.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Element Compound Magnesium is a group two element and is the eighth most common element in the earth's crust. The first question we ask is if the compound is ionic or covalent? A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. The ions play a major role in. Sources,. Magnesium Ion Element Compound.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Element Compound Its compounds are widely used in. That is, does it have ionic bonds, or covalent bonds? The first question we ask is if the compound is ionic or covalent? Magnesium is a group two element and is the eighth most common element in the earth's crust. Its ions are essential to all living cells. The ions play a major role. Magnesium Ion Element Compound.
From valenceelectrons.com
Electron Configuration Magnesium Ion Element Compound Its ions are essential to all living cells. That is, does it have ionic bonds, or covalent bonds? A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Its compounds are widely used in. Magnesium typically forms ionic bonds, owing to its two valence electrons. Sources, facts, uses,. Magnesium Ion Element Compound.
From utedzz.blogspot.com
Periodic Table Magnesium Element Periodic Table Timeline Magnesium Ion Element Compound Magnesium typically forms ionic bonds, owing to its two valence electrons. The first question we ask is if the compound is ionic or covalent? Sources, facts, uses, scarcity (sri), podcasts, alchemical. Its ions are essential to all living cells. Magnesium is the 11th most abundant element by mass in the human body. That is, does it have ionic bonds, or. Magnesium Ion Element Compound.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Magnesium Ion Element Compound Magnesium is the 11th most abundant element by mass in the human body. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Its compounds are widely used in. Magnesium typically forms ionic bonds, owing to its two valence electrons. Its ions are essential to all living cells. Magnesium is a group two element and is the eighth most common element in the. Magnesium Ion Element Compound.
From montessorimuddle.org
subatomic particles Montessori Muddle Magnesium Ion Element Compound The first question we ask is if the compound is ionic or covalent? The ions play a major role in. Its ions are essential to all living cells. Magnesium is the 11th most abundant element by mass in the human body. Its compounds are widely used in. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium typically forms ionic bonds, owing. Magnesium Ion Element Compound.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Ion Element Compound The ions play a major role in. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Magnesium is the 11th most abundant element by mass in the human body. Magnesium is a group two element and is the eighth most common element in the earth's crust. That is, does it have ionic bonds, or covalent bonds? A magnesium atom must lose two. Magnesium Ion Element Compound.