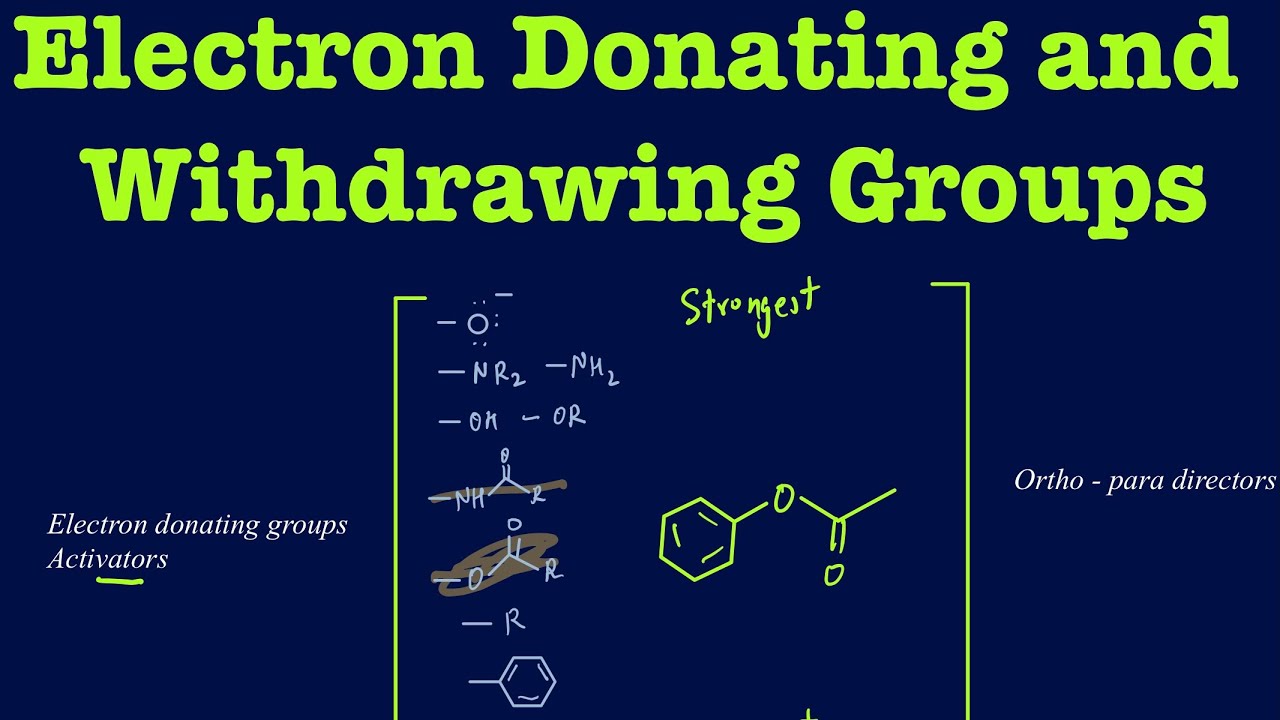
What Do Electron Withdrawing Groups Do . Activating groups increase the rate. Deactivating groups decrease the rate. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg = electron donating group. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Compare the data in the table below. For electron withdrawing groups, all of the sigma complexes are destabilized.
from www.youtube.com
Edg = electron donating group. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Compare the data in the table below. For electron withdrawing groups, all of the sigma complexes are destabilized. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Deactivating groups decrease the rate. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Activating groups increase the rate.
Summary of electron.donating and withdrawing groups. Reactivity toward
What Do Electron Withdrawing Groups Do Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Deactivating groups decrease the rate. Edg = electron donating group. For electron withdrawing groups, all of the sigma complexes are destabilized. Activating groups increase the rate. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Compare the data in the table below.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations What Do Electron Withdrawing Groups Do Deactivating groups decrease the rate. For electron withdrawing groups, all of the sigma complexes are destabilized. Edg = electron donating group. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Compare the data in the table below. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Activating groups increase the rate. Groups like. What Do Electron Withdrawing Groups Do.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network What Do Electron Withdrawing Groups Do Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Edg = electron donating group. Deactivating groups decrease the rate. Compare the data in the table below. Activating groups increase the rate. Groups like or and nr 2 that seem like they should be deactivating. What Do Electron Withdrawing Groups Do.
From www.slideserve.com
PPT Electrophilic Aromatic Substitution PowerPoint Presentation, free What Do Electron Withdrawing Groups Do Deactivating groups decrease the rate. For electron withdrawing groups, all of the sigma complexes are destabilized. Compare the data in the table below. Activating groups increase the rate. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Edg = electron donating group. Fluoroacetate anion stabilized by electron withdrawing. What Do Electron Withdrawing Groups Do.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep What Do Electron Withdrawing Groups Do Edg = electron donating group. For electron withdrawing groups, all of the sigma complexes are destabilized. Activating groups increase the rate. Deactivating groups decrease the rate. Compare the data in the table below. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Groups like. What Do Electron Withdrawing Groups Do.
From www.youtube.com
Why nitro group is electron withdrawing? YouTube What Do Electron Withdrawing Groups Do Compare the data in the table below. Activating groups increase the rate. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Deactivating groups decrease the rate. For electron withdrawing groups, all of the sigma complexes are destabilized. Edg = electron donating group. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity. What Do Electron Withdrawing Groups Do.
From www.masterorganicchemistry.com
Acidity Trends In Organic Chemistry Master Organic Chemistry What Do Electron Withdrawing Groups Do Edg can be recognised by lone pairs on the atom adjacent to the π system,. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Deactivating groups decrease the rate. For electron withdrawing groups, all of the sigma complexes are destabilized. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually. What Do Electron Withdrawing Groups Do.
From www.youtube.com
EAS Electron withdrawing group regiochem YouTube What Do Electron Withdrawing Groups Do Edg can be recognised by lone pairs on the atom adjacent to the π system,. Compare the data in the table below. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Deactivating groups decrease the rate. Activating groups increase the rate. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are. What Do Electron Withdrawing Groups Do.
From www.researchgate.net
The effect of electronwithdrawing and electrondonating groups on What Do Electron Withdrawing Groups Do For electron withdrawing groups, all of the sigma complexes are destabilized. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Activating groups increase the rate. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually. What Do Electron Withdrawing Groups Do.
From brainly.in
Electron withdrawing and donating groups list Brainly.in What Do Electron Withdrawing Groups Do Compare the data in the table below. Activating groups increase the rate. Edg = electron donating group. Deactivating groups decrease the rate. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Groups like or and nr 2 that seem like they should be deactivating. What Do Electron Withdrawing Groups Do.
From openpress.usask.ca
10.10. Regioselectivity and Substituent Effects Introduction to What Do Electron Withdrawing Groups Do For electron withdrawing groups, all of the sigma complexes are destabilized. Edg = electron donating group. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Compare the data in the table below. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Activating. What Do Electron Withdrawing Groups Do.
From www.youtube.com
electronwithdrawing groups YouTube What Do Electron Withdrawing Groups Do Compare the data in the table below. Edg = electron donating group. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Activating groups increase the rate. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Edg can be recognised by lone pairs on the atom adjacent to. What Do Electron Withdrawing Groups Do.
From www.youtube.com
30.04 Electrondonating and withdrawing Groups YouTube What Do Electron Withdrawing Groups Do Deactivating groups decrease the rate. For electron withdrawing groups, all of the sigma complexes are destabilized. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Edg = electron donating group. Edg can be recognised by lone pairs on. What Do Electron Withdrawing Groups Do.
From www.slideserve.com
PPT Chapter 2 Phenols PowerPoint Presentation, free download ID2962623 What Do Electron Withdrawing Groups Do Deactivating groups decrease the rate. Compare the data in the table below. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Activating groups increase the rate. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are. What Do Electron Withdrawing Groups Do.
From www.chemistrysteps.com
Basicity of Amines Chemistry Steps What Do Electron Withdrawing Groups Do For electron withdrawing groups, all of the sigma complexes are destabilized. Compare the data in the table below. Deactivating groups decrease the rate. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Activating groups increase the rate. Edg = electron donating group. Groups like or and nr 2 that seem like they should be. What Do Electron Withdrawing Groups Do.
From chem.libretexts.org
23.1 Properties of amines Chemistry LibreTexts What Do Electron Withdrawing Groups Do Compare the data in the table below. Edg = electron donating group. Activating groups increase the rate. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Deactivating groups decrease the rate. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Fluoroacetate anion. What Do Electron Withdrawing Groups Do.
From www.youtube.com
Electron Withdrawing & Donating Groups Polarity YouTube What Do Electron Withdrawing Groups Do Activating groups increase the rate. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg = electron donating group. Compare the data in the table below. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Deactivating groups decrease the rate. For electron withdrawing groups, all of the. What Do Electron Withdrawing Groups Do.
From www.researchgate.net
Influence of an electronwithdrawing group (EWG) and cycle strain on What Do Electron Withdrawing Groups Do Compare the data in the table below. Deactivating groups decrease the rate. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Activating groups increase the rate. Edg = electron donating group. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. For electron. What Do Electron Withdrawing Groups Do.
From quizlet.com
Electron Withdrawing Groups Diagram Quizlet What Do Electron Withdrawing Groups Do Compare the data in the table below. Deactivating groups decrease the rate. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. For electron withdrawing groups, all of the sigma complexes are destabilized. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Activating groups increase the rate. Edg = electron donating group. Groups like. What Do Electron Withdrawing Groups Do.
From www.organicchemistrytutor.com
Directing Effects in Electrophilic Aromatic Substitution Reactions What Do Electron Withdrawing Groups Do Edg can be recognised by lone pairs on the atom adjacent to the π system,. Activating groups increase the rate. Edg = electron donating group. Compare the data in the table below. Deactivating groups decrease the rate. For electron withdrawing groups, all of the sigma complexes are destabilized. Groups like or and nr 2 that seem like they should be. What Do Electron Withdrawing Groups Do.
From www.scribd.com
Electron Withdrawing and Electron Donating Groups Functional Group Ion What Do Electron Withdrawing Groups Do For electron withdrawing groups, all of the sigma complexes are destabilized. Activating groups increase the rate. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Edg = electron donating group. Compare the data in the table below. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Deactivating groups decrease the rate. Groups like. What Do Electron Withdrawing Groups Do.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network What Do Electron Withdrawing Groups Do Compare the data in the table below. Activating groups increase the rate. Deactivating groups decrease the rate. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. For electron withdrawing groups, all of the sigma complexes are destabilized. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Groups like or and nr 2 that. What Do Electron Withdrawing Groups Do.
From byjus.com
Do electron withdrawing group increases reactivity towards nucleophilic What Do Electron Withdrawing Groups Do Edg can be recognised by lone pairs on the atom adjacent to the π system,. Activating groups increase the rate. Compare the data in the table below. Deactivating groups decrease the rate. Edg = electron donating group. For electron withdrawing groups, all of the sigma complexes are destabilized. Groups like or and nr 2 that seem like they should be. What Do Electron Withdrawing Groups Do.
From www.youtube.com
Summary of electron.donating and withdrawing groups. Reactivity toward What Do Electron Withdrawing Groups Do Edg = electron donating group. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Deactivating groups decrease the rate. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Compare the data in. What Do Electron Withdrawing Groups Do.
From electronic--racket.blogspot.com
How To Identify Electron Withdrawing And Donating Groups What Do Electron Withdrawing Groups Do Compare the data in the table below. For electron withdrawing groups, all of the sigma complexes are destabilized. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg = electron donating group. Deactivating groups decrease the rate. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Edg. What Do Electron Withdrawing Groups Do.
From byjus.com
Why does electron withdrawing group increases the acidity and releasing What Do Electron Withdrawing Groups Do For electron withdrawing groups, all of the sigma complexes are destabilized. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Edg = electron donating group. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually. What Do Electron Withdrawing Groups Do.
From www.chegg.com
Solved 3. The nitro group (NO2) is an electronwithdrawing What Do Electron Withdrawing Groups Do Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Activating groups increase the rate. Compare the data in the table below. Deactivating groups decrease the rate. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are. What Do Electron Withdrawing Groups Do.
From www.numerade.com
SOLVED ElectronWithdrawing Groups in Carbonyls One other thing to What Do Electron Withdrawing Groups Do Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. For electron withdrawing groups, all of the sigma complexes are destabilized. Edg = electron donating group. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Activating groups increase the rate. Deactivating groups decrease the rate. Edg can be. What Do Electron Withdrawing Groups Do.
From byjus.com
How does adding an electron withdrawing group to buy benzene diazonium What Do Electron Withdrawing Groups Do For electron withdrawing groups, all of the sigma complexes are destabilized. Compare the data in the table below. Edg = electron donating group. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Fluoroacetate. What Do Electron Withdrawing Groups Do.
From www.pinterest.com
صورة ذات صلة Organic chemistry, Organic chem, Chemistry What Do Electron Withdrawing Groups Do Edg = electron donating group. Compare the data in the table below. Activating groups increase the rate. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Deactivating groups decrease the rate. For electron withdrawing groups, all of the sigma complexes are destabilized. Groups like. What Do Electron Withdrawing Groups Do.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep What Do Electron Withdrawing Groups Do Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Deactivating groups decrease the rate. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Activating groups increase the rate. For electron withdrawing groups, all of the sigma complexes are destabilized. Edg can be recognised by lone pairs on. What Do Electron Withdrawing Groups Do.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network What Do Electron Withdrawing Groups Do Deactivating groups decrease the rate. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. For electron withdrawing groups, all of the sigma complexes are destabilized. Compare the data in the table below. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Activating groups increase the rate. Edg = electron donating group. Groups like. What Do Electron Withdrawing Groups Do.
From byjus.com
26.how does the attachment of an electron withdrawing group like NO2 in What Do Electron Withdrawing Groups Do Activating groups increase the rate. Compare the data in the table below. For electron withdrawing groups, all of the sigma complexes are destabilized. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Deactivating groups decrease the rate. Edg = electron donating group. Groups like. What Do Electron Withdrawing Groups Do.
From www.slideserve.com
PPT Chapter 2 Phenols PowerPoint Presentation, free download ID2962623 What Do Electron Withdrawing Groups Do For electron withdrawing groups, all of the sigma complexes are destabilized. Deactivating groups decrease the rate. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Compare the data in the table below. Edg = electron donating group. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Groups like or and nr 2 that. What Do Electron Withdrawing Groups Do.
From www.youtube.com
What is Electron Withdrawing Groups ? Inductive effect & Acidity What Do Electron Withdrawing Groups Do Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. Fluoroacetate anion stabilized by electron withdrawing inductive effect of fluorine. Edg = electron donating group. Deactivating groups decrease the rate. Activating groups increase the rate. Compare the data in the table below. Edg can be recognised by lone pairs. What Do Electron Withdrawing Groups Do.
From www.masterorganicchemistry.com
Nucleophilicity Trends of Amines Master Organic Chemistry What Do Electron Withdrawing Groups Do Edg = electron donating group. Edg can be recognised by lone pairs on the atom adjacent to the π system,. Groups like or and nr 2 that seem like they should be deactivating because of their electronegativity are actually activating since. For electron withdrawing groups, all of the sigma complexes are destabilized. Compare the data in the table below. Fluoroacetate. What Do Electron Withdrawing Groups Do.