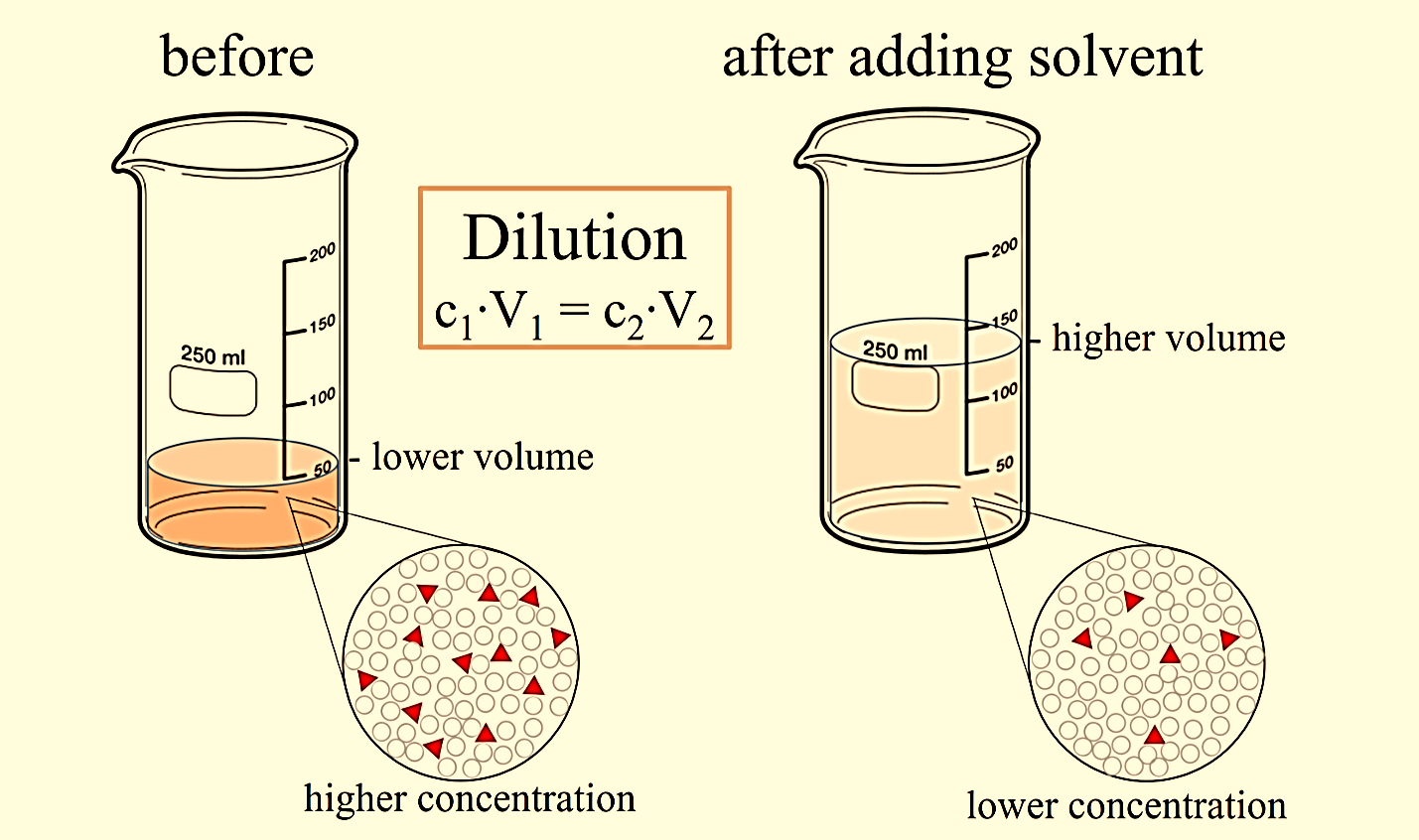
How Would You Produce A 10^-1 Dilution Of A 3 Ml . If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. The diluted liquid needs to be thoroughly mixed to achieve true dilution. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Our expert help has broken down your problem into an.
from www.w3schools.blog
If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Our expert help has broken down your problem into an. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. The diluted liquid needs to be thoroughly mixed to achieve true dilution. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go!
Standard enthalpy of dilution W3schools
How Would You Produce A 10^-1 Dilution Of A 3 Ml Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. The diluted liquid needs to be thoroughly mixed to achieve true dilution. Our expert help has broken down your problem into an.
From www.youtube.com
U10L4 Molarity, Dilution, PPM, and Molality Calculations YouTube How Would You Produce A 10^-1 Dilution Of A 3 Ml Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. The diluted liquid needs. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.chegg.com
Solved would you produce a 101 dilution of a 3 ml. How Would You Produce A 10^-1 Dilution Of A 3 Ml The diluted liquid needs to be thoroughly mixed to achieve true dilution. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. Our expert help has broken down your problem into an.. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.numerade.com
SOLVED How would you produce a 10^1 dilution of a 3 mL bacterial How Would You Produce A 10^-1 Dilution Of A 3 Ml The diluted liquid needs to be thoroughly mixed to achieve true dilution. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you wish to perform dilution factor or fold dilution calculations for solutions. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in How Would You Produce A 10^-1 Dilution Of A 3 Ml The diluted liquid needs to be thoroughly mixed to achieve true dilution. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you wish to perform dilution factor or fold dilution calculations for solutions. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.chegg.com
Solved Use the dilution scheme shown below and answer the How Would You Produce A 10^-1 Dilution Of A 3 Ml Our expert help has broken down your problem into an. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. Total dilution factor for the second tube = dilution of first. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual How Would You Produce A 10^-1 Dilution Of A 3 Ml Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. You have 0.3. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.chegg.com
Solved 3 How would you produce a 101 dilution of a 3 ml How Would You Produce A 10^-1 Dilution Of A 3 Ml Our expert help has broken down your problem into an. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. The diluted liquid needs to be thoroughly mixed to achieve true dilution. If you. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY How Would You Produce A 10^-1 Dilution Of A 3 Ml If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Our expert help has. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems How Would You Produce A 10^-1 Dilution Of A 3 Ml You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. The diluted liquid needs. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.numerade.com
SOLVED "Iow would you produce a 10' dilution of a 3 mL bacterial How Would You Produce A 10^-1 Dilution Of A 3 Ml If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. The diluted liquid needs to be thoroughly mixed to achieve true dilution. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Our expert help has broken down your problem into. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.numerade.com
SOLVED 1. How would you produce a 10^1 dilution of a 3 mL bacterial How Would You Produce A 10^-1 Dilution Of A 3 Ml If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. Our expert help has broken down your problem into an. The diluted liquid needs to be thoroughly mixed to achieve true dilution. If you. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From bio.libretexts.org
1.8 Serial Dilutions and Standard Curve Biology LibreTexts How Would You Produce A 10^-1 Dilution Of A 3 Ml If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. The diluted liquid needs to be thoroughly mixed to achieve true dilution. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Total dilution factor for the second tube = dilution. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.youtube.com
Dilution Calculations for Molecular Biology YouTube How Would You Produce A 10^-1 Dilution Of A 3 Ml You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Our expert help has broken down your problem into an. The diluted liquid needs to be thoroughly mixed to achieve true dilution. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. If you wish. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.w3schools.blog
Standard enthalpy of dilution W3schools How Would You Produce A 10^-1 Dilution Of A 3 Ml Our expert help has broken down your problem into an. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. The diluted liquid needs to be thoroughly mixed to achieve true dilution. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you wish. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From mungfali.com
10 Fold Serial Dilution How Would You Produce A 10^-1 Dilution Of A 3 Ml The diluted liquid needs to be thoroughly mixed to achieve true dilution. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Our expert help has broken down your problem into an. If you. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality How Would You Produce A 10^-1 Dilution Of A 3 Ml Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. The diluted liquid needs to be thoroughly. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory How Would You Produce A 10^-1 Dilution Of A 3 Ml If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. The diluted liquid needs to be thoroughly mixed to achieve true dilution. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From zhtutorials.com
Serial Dilution Practical Skills Ep 3 Zoë Huggett Tutorials How Would You Produce A 10^-1 Dilution Of A 3 Ml You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. The diluted liquid needs to be thoroughly mixed to achieve true dilution. Total dilution factor for the second tube = dilution of. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.chegg.com
Solved 3 How would you produce a 104 dilution of a 3 mL How Would You Produce A 10^-1 Dilution Of A 3 Ml The diluted liquid needs to be thoroughly mixed to achieve true dilution. Our expert help has broken down your problem into an. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew How Would You Produce A 10^-1 Dilution Of A 3 Ml You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. The diluted liquid needs to be thoroughly mixed to achieve true dilution. If you wish to perform dilution factor or fold dilution calculations for solutions. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.chegg.com
3 How would you produce a 10−1 dilution of a 3 mL How Would You Produce A 10^-1 Dilution Of A 3 Ml If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. Our expert help has broken down your problem into an. You have 0.3 ml of an undiluted culture at a density of your solution’s. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems How Would You Produce A 10^-1 Dilution Of A 3 Ml You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Our expert help has broken down your problem into an. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.youtube.com
Molarity and Dilution YouTube How Would You Produce A 10^-1 Dilution Of A 3 Ml Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. Our expert help has broken down your problem into an. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. The diluted liquid needs to be thoroughly mixed to achieve true. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.chegg.com
3 How would yои produce a 10−1 dilution of a 3 mL How Would You Produce A 10^-1 Dilution Of A 3 Ml You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. Our expert help has broken down your problem into an. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.vrogue.co
10 Examples Of Solutions vrogue.co How Would You Produce A 10^-1 Dilution Of A 3 Ml Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. The diluted liquid needs to be thoroughly mixed to achieve true dilution. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. If you have a 1:3 dilution, i.e., a 1:3. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina How Would You Produce A 10^-1 Dilution Of A 3 Ml If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Our expert help has broken down your. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.youtube.com
Dilution, solution, ratio, proportion (laboratory. calculations) YouTube How Would You Produce A 10^-1 Dilution Of A 3 Ml You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. Our expert help has broken down your. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.chegg.com
3 How would yои produce a 10−1 dilution of a 3 mL How Would You Produce A 10^-1 Dilution Of A 3 Ml If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. The diluted liquid needs to be thoroughly mixed to achieve true dilution. If you wish to perform dilution factor or fold dilution calculations for. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.chegg.com
Solved 3 How would you produce a 10−1 dilution of a 3 mL How Would You Produce A 10^-1 Dilution Of A 3 Ml Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Our expert help has broken down your problem into an. The diluted liquid needs to be thoroughly mixed to achieve true dilution. If you have. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.chegg.com
Solved Practice Dilution Problems Section I 1. How much How Would You Produce A 10^-1 Dilution Of A 3 Ml Our expert help has broken down your problem into an. Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.medicine.mcgill.ca
Serial Dilutions How Would You Produce A 10^-1 Dilution Of A 3 Ml Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. Our expert help has broken down your problem into an. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. You have 0.3 ml of an undiluted culture at a density. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.youtube.com
How to Calculate Dilution Factor YouTube How Would You Produce A 10^-1 Dilution Of A 3 Ml Our expert help has broken down your problem into an. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. Total dilution factor for the second tube = dilution of first. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube How Would You Produce A 10^-1 Dilution Of A 3 Ml The diluted liquid needs to be thoroughly mixed to achieve true dilution. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. If you wish to perform dilution factor or fold dilution calculations for solutions. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From bdaschools.weebly.com
bdaschools Blog How Would You Produce A 10^-1 Dilution Of A 3 Ml Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! The diluted liquid needs to be thoroughly. How Would You Produce A 10^-1 Dilution Of A 3 Ml.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision How Would You Produce A 10^-1 Dilution Of A 3 Ml If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit. You have 0.3 ml of an undiluted culture at a density of your solution’s ready to go! Total dilution factor for the second tube = dilution of first tube × dilution of the second tube. The diluted liquid needs to be thoroughly. How Would You Produce A 10^-1 Dilution Of A 3 Ml.