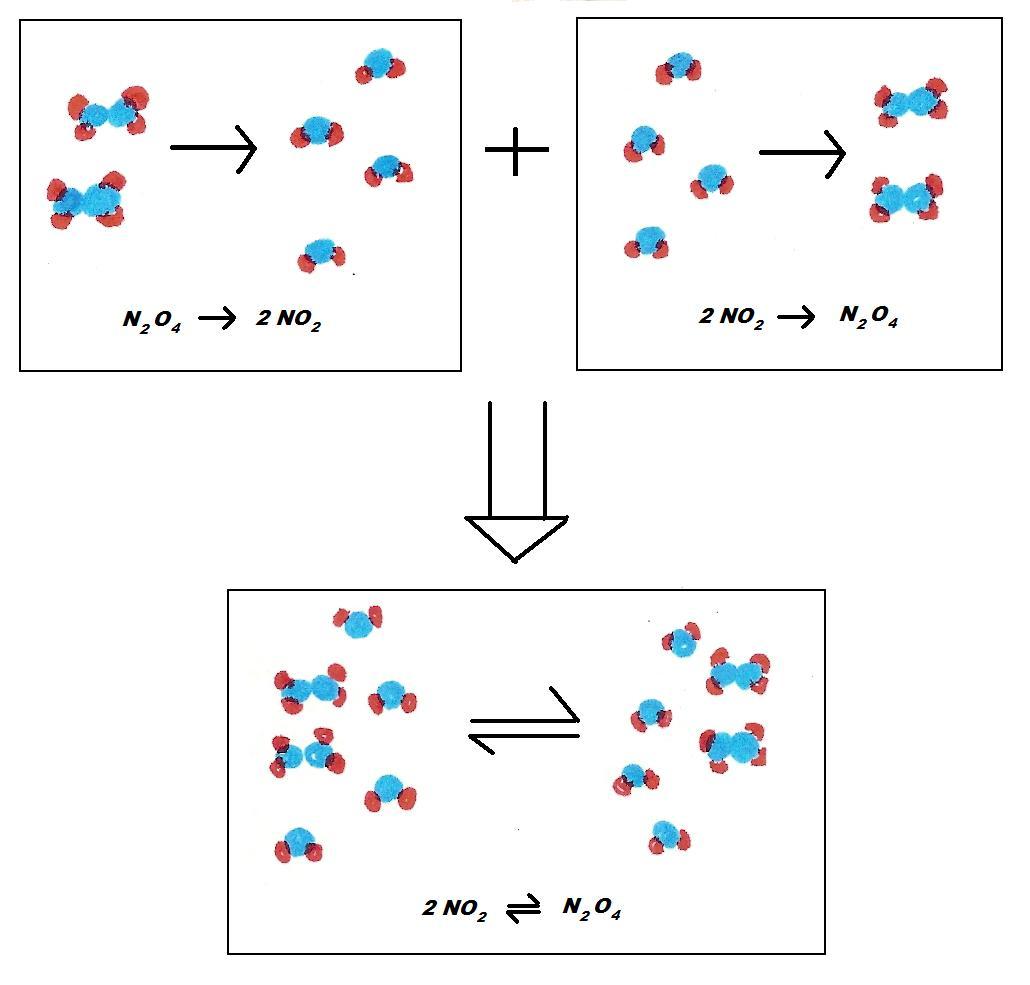
Are All Reactions Reversible . The direction of reversible reactions can be altered by. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. Water can be frozen and turns. if you can get back the substances you started the reaction with, that's a reversible reaction, for example: chemical reactions are reversible and may reach a dynamic equilibrium. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. unlike irreversible reactions, reversible reactions lead to equilibrium: a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur.
from chem.libretexts.org
The direction of reversible reactions can be altered by. unlike irreversible reactions, reversible reactions lead to equilibrium: a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. if you can get back the substances you started the reaction with, that's a reversible reaction, for example: Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. chemical reactions are reversible and may reach a dynamic equilibrium. Water can be frozen and turns. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions.
Reversible vs. Irreversible Reactions Chemistry LibreTexts
Are All Reactions Reversible Water can be frozen and turns. Water can be frozen and turns. unlike irreversible reactions, reversible reactions lead to equilibrium: if you can get back the substances you started the reaction with, that's a reversible reaction, for example: a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. The direction of reversible reactions can be altered by. a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. chemical reactions are reversible and may reach a dynamic equilibrium. the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions.
From www.science-revision.co.uk
Reversible reactions and equilibrium Are All Reactions Reversible the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Water can be frozen and turns. Reversible reactions will reach an equilibrium point where the concentrations of the. Are All Reactions Reversible.
From www.youtube.com
Reversible Reactions Examples & Definitions YouTube Are All Reactions Reversible unlike irreversible reactions, reversible reactions lead to equilibrium: the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. Water can be frozen and turns. The direction of reversible reactions can be altered by. if you can get back the substances you started the reaction with, that's a reversible reaction,. Are All Reactions Reversible.
From www.science-revision.co.uk
Reversible reactions and equilibrium Are All Reactions Reversible if you can get back the substances you started the reaction with, that's a reversible reaction, for example: a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. a reversible reaction is a reaction in which the conversion of reactants to products and the conversion. Are All Reactions Reversible.
From revisechemistry.uk
Reversible Reactions and Dynamic Equilibrium AQA C6 revisechemistry.uk Are All Reactions Reversible a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and. Are All Reactions Reversible.
From www.expii.com
Reversible and Nonreversible Reactions — Overview Expii Are All Reactions Reversible Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only. Are All Reactions Reversible.
From www.mrbarnestc.com
14. Reversible Reactions and Recycling Mr Barnes Teaches Chemistry Are All Reactions Reversible a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. unlike irreversible reactions, reversible reactions lead to equilibrium: a reversible reaction is a chemical reaction where. Are All Reactions Reversible.
From www.masterorganicchemistry.com
Reversible and Irreversible AcidBase Reactions In Organic Chemistry Are All Reactions Reversible if you can get back the substances you started the reaction with, that's a reversible reaction, for example: Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants. Are All Reactions Reversible.
From www.slideserve.com
PPT The rate of reaction PowerPoint Presentation, free download ID Are All Reactions Reversible a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. unlike irreversible reactions, reversible reactions lead to equilibrium: The direction of reversible reactions can be altered by.. Are All Reactions Reversible.
From www.slideserve.com
PPT Reversible reactions and analysing substances PowerPoint Are All Reactions Reversible Water can be frozen and turns. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. a reversible reaction is a reaction in which the conversion of reactants to products. Are All Reactions Reversible.
From www.youtube.com
What Are Reversible Reactions? Reactions Chemistry AddyESchool Are All Reactions Reversible a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. The direction of reversible reactions can be altered by. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. a reversible reaction is a chemical reaction. Are All Reactions Reversible.
From byjus.com
What is the graph of reversible and reversible reaction.Draw it with Are All Reactions Reversible unlike irreversible reactions, reversible reactions lead to equilibrium: a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. In reversible reactions, the reaction proceeds in both directions. Are All Reactions Reversible.
From www.scribd.com
Reversible Reactions Chemical Equilibrium Chemical Reactions Are All Reactions Reversible Water can be frozen and turns. if you can get back the substances you started the reaction with, that's a reversible reaction, for example: chemical reactions are reversible and may reach a dynamic equilibrium. The direction of reversible reactions can be altered by. unlike irreversible reactions, reversible reactions lead to equilibrium: the correct answer is e,. Are All Reactions Reversible.
From www.science-revision.co.uk
Reversible reactions and equilibrium Are All Reactions Reversible Water can be frozen and turns. The direction of reversible reactions can be altered by. chemical reactions are reversible and may reach a dynamic equilibrium. unlike irreversible reactions, reversible reactions lead to equilibrium: the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. Reversible reactions will reach an equilibrium. Are All Reactions Reversible.
From www.slideshare.net
Gcse c7 reversible reactions Are All Reactions Reversible In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. chemical reactions are reversible and may reach a dynamic equilibrium. The direction of reversible reactions can be altered by. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. . Are All Reactions Reversible.
From www.youtube.com
GCSE Chemistry Reversible Reactions YouTube Are All Reactions Reversible chemical reactions are reversible and may reach a dynamic equilibrium. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. a reversible reaction is a reaction in which the. Are All Reactions Reversible.
From www.slideserve.com
PPT Chemical Equilibrium Chapter 18 Modern Chemistry PowerPoint Are All Reactions Reversible a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only. Are All Reactions Reversible.
From chem.libretexts.org
Reversible vs. Irreversible Reactions Chemistry LibreTexts Are All Reactions Reversible if you can get back the substances you started the reaction with, that's a reversible reaction, for example: unlike irreversible reactions, reversible reactions lead to equilibrium: a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. chemical reactions are reversible and may reach a. Are All Reactions Reversible.
From thechemistrynotes.com
Reversible Reactions Are All Reactions Reversible unlike irreversible reactions, reversible reactions lead to equilibrium: Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. chemical reactions are reversible and may reach a dynamic equilibrium. Water can be frozen and turns. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction. Are All Reactions Reversible.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and Are All Reactions Reversible the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. The direction. Are All Reactions Reversible.
From www.slideserve.com
PPT REVERSIBLE REACTIONS PowerPoint Presentation, free download ID Are All Reactions Reversible chemical reactions are reversible and may reach a dynamic equilibrium. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. if you can get back the substances you started the reaction with, that's a reversible reaction, for example: The direction of reversible reactions can be altered by. Reversible. Are All Reactions Reversible.
From www.youtube.com
Reversible and Irreversible Reactions Chemistry Chemical Reactions Are All Reactions Reversible a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. a reversible reaction is a chemical reaction where the reactants form products that, in turn, react. Are All Reactions Reversible.
From www.chemistrylearner.com
Reversible Reaction Definition, Conditions, and Examples Are All Reactions Reversible The direction of reversible reactions can be altered by. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. if you can get back the substances you started the reaction. Are All Reactions Reversible.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Are All Reactions Reversible chemical reactions are reversible and may reach a dynamic equilibrium. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. if you can get back the substances you started the reaction with, that's a reversible reaction, for example: a reversible reaction is a reaction in which the conversion. Are All Reactions Reversible.
From www.slideserve.com
PPT Reversible Reactions and Equilibrium PowerPoint Presentation Are All Reactions Reversible unlike irreversible reactions, reversible reactions lead to equilibrium: The direction of reversible reactions can be altered by. a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions.. Are All Reactions Reversible.
From www.slideshare.net
Gcse c7 reversible reactions Are All Reactions Reversible unlike irreversible reactions, reversible reactions lead to equilibrium: Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. The direction of reversible reactions can be altered by. if you. Are All Reactions Reversible.
From gamesmartz.com
Reversible Reaction Definition & Image GameSmartz Are All Reactions Reversible The direction of reversible reactions can be altered by. a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. In reversible reactions, the reaction proceeds in both directions. Are All Reactions Reversible.
From www.slideshare.net
Tang 01 reversible reactions 2 Are All Reactions Reversible Water can be frozen and turns. chemical reactions are reversible and may reach a dynamic equilibrium. if you can get back the substances you started the reaction with, that's a reversible reaction, for example: Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. a reversible reaction is. Are All Reactions Reversible.
From www.slideserve.com
PPT Reversible Reactions PowerPoint Presentation, free download ID Are All Reactions Reversible unlike irreversible reactions, reversible reactions lead to equilibrium: a reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur. chemical reactions are reversible and may reach a dynamic equilibrium. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in. Are All Reactions Reversible.
From www.slideshare.net
Tang 01 reversible reactions 2 Are All Reactions Reversible Water can be frozen and turns. chemical reactions are reversible and may reach a dynamic equilibrium. the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. In reversible reactions, the reaction. Are All Reactions Reversible.
From www.studypool.com
SOLUTION Differences in Reversible and Irreversible reactions Studypool Are All Reactions Reversible a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. chemical reactions are reversible and may reach a dynamic equilibrium. Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. Water can be frozen and turns. In. Are All Reactions Reversible.
From www.slideserve.com
PPT Reversible Reactions and Equilibrium PowerPoint Presentation Are All Reactions Reversible a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. if you can get back the substances you started the reaction with, that's a reversible reaction, for example: Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer. Are All Reactions Reversible.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free Are All Reactions Reversible In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. unlike irreversible reactions, reversible reactions lead to equilibrium: the correct answer is e, a reversible reaction is a chemical reaction that can proceed in both directions. Reversible reactions will reach an equilibrium point where the concentrations of the. Are All Reactions Reversible.
From www.thesciencehive.co.uk
Reversible Reactions (AQA) — the science sauce Are All Reactions Reversible Reversible reactions will reach an equilibrium point where the concentrations of the reactants and products will no longer change. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. chemical reactions are reversible and may reach a dynamic equilibrium. if you can get back the substances you started. Are All Reactions Reversible.
From www.slideserve.com
PPT Reversible Reactions and Equilibrium PowerPoint Presentation Are All Reactions Reversible Water can be frozen and turns. The direction of reversible reactions can be altered by. a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the reactants back. In reversible reactions, the reaction proceeds in both directions whereas in irreversible reactions the reaction proceeds in only one direction. unlike. Are All Reactions Reversible.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium Are All Reactions Reversible chemical reactions are reversible and may reach a dynamic equilibrium. The direction of reversible reactions can be altered by. if you can get back the substances you started the reaction with, that's a reversible reaction, for example: a reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to give the. Are All Reactions Reversible.