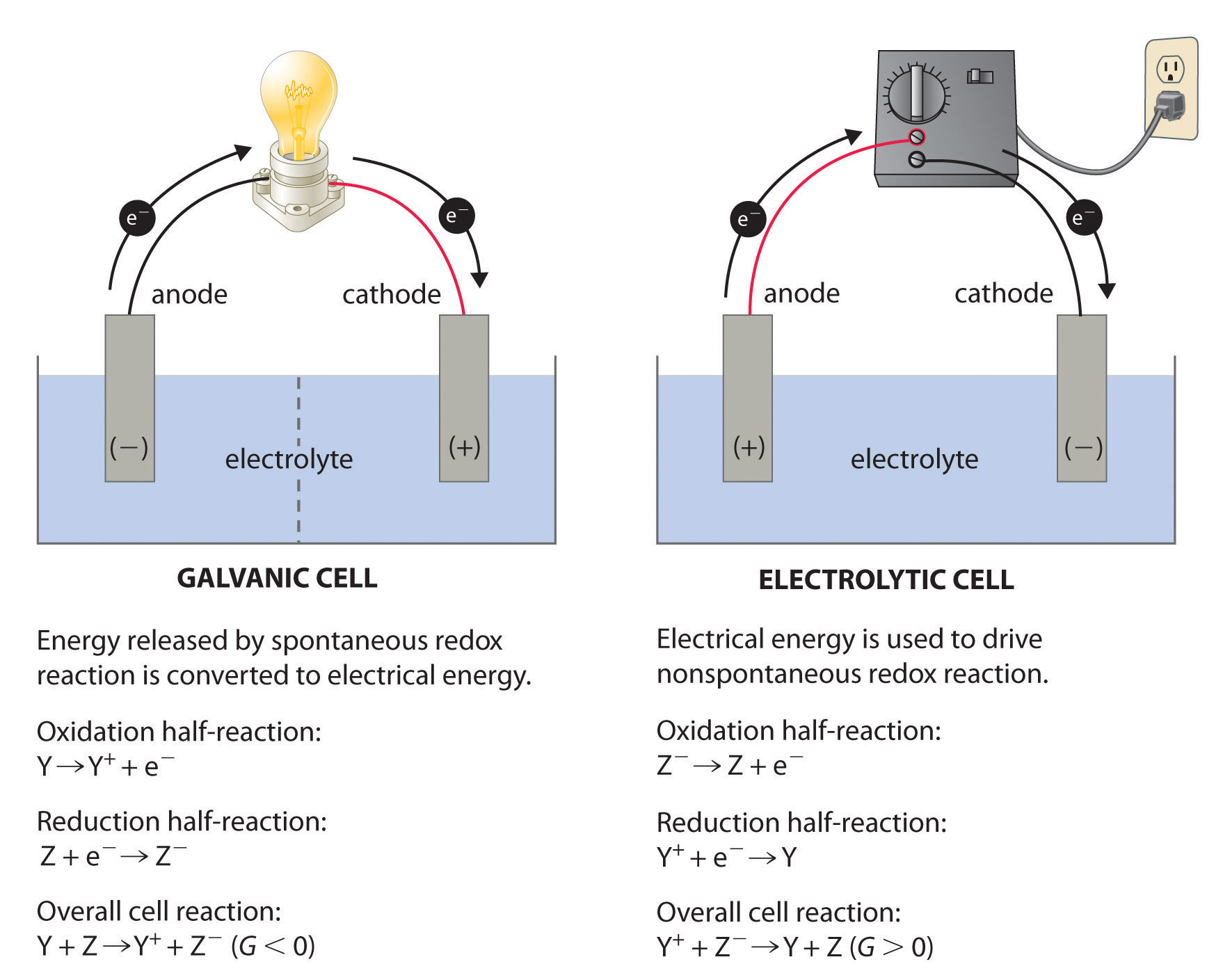
Electrochemical Cells And Cell Potentials . Interpret electrode potentials in terms of relative oxidant and. Calculate cell potentials and predict redox spontaneity using standard electrode potentials Calculate cell potentials and predict redox spontaneity using standard electrode potentials describe and relate the definitions of electrode and cell potentials. Electrons are able to move between electrodes because the chemical reaction is a redox reaction. describe and relate the definitions of electrode and cell potentials; the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant strengths; electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. describe and relate the definitions of electrode and cell potentials; Interpret electrode potentials in terms of relative oxidant and reductant strengths; The potential difference is caused by the ability of electrons to flow from one half cell to the other.
from chem.libretexts.org
Interpret electrode potentials in terms of relative oxidant and reductant strengths; Electrons are able to move between electrodes because the chemical reaction is a redox reaction. Calculate cell potentials and predict redox spontaneity using standard electrode potentials describe and relate the definitions of electrode and cell potentials. electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Interpret electrode potentials in terms of relative oxidant and reductant strengths; in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. Calculate cell potentials and predict redox spontaneity using standard electrode potentials describe and relate the definitions of electrode and cell potentials; the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell.
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts
Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials. in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. Interpret electrode potentials in terms of relative oxidant and reductant strengths; The potential difference is caused by the ability of electrons to flow from one half cell to the other. electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant strengths; describe and relate the definitions of electrode and cell potentials; Calculate cell potentials and predict redox spontaneity using standard electrode potentials describe and relate the definitions of electrode and cell potentials; Interpret electrode potentials in terms of relative oxidant and. Calculate cell potentials and predict redox spontaneity using standard electrode potentials Electrons are able to move between electrodes because the chemical reaction is a redox reaction. describe and relate the definitions of electrode and cell potentials.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Electrochemical Cells And Cell Potentials the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. describe and relate the definitions of electrode and cell potentials; Interpret electrode potentials in terms of relative oxidant and reductant strengths; in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using. Electrochemical Cells And Cell Potentials.
From www.studypool.com
SOLUTION CHEM151 Phoenix Electrochemical Cells and Cell Potentials Lab Electrochemical Cells And Cell Potentials The potential difference is caused by the ability of electrons to flow from one half cell to the other. Interpret electrode potentials in terms of relative oxidant and. Calculate cell potentials and predict redox spontaneity using standard electrode potentials Interpret electrode potentials in terms of relative oxidant and reductant strengths; Calculate cell potentials and predict redox spontaneity using standard electrode. Electrochemical Cells And Cell Potentials.
From www.youtube.com
AQA 1.11 Electrode Potentials and Electrochemical Cells REVISION YouTube Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials; The potential difference is caused by the ability of electrons to flow from one half cell to the other. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant. Electrochemical Cells And Cell Potentials.
From chem.libretexts.org
19.5 Standard Electrochemical Potentials Chemistry LibreTexts Electrochemical Cells And Cell Potentials the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons.. Electrochemical Cells And Cell Potentials.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials; electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Electrons are able to move between electrodes because the chemical reaction is a redox reaction. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in. Electrochemical Cells And Cell Potentials.
From guidepartgail.z21.web.core.windows.net
Electric Cell Diagram Notation Electrochemical Cells And Cell Potentials Calculate cell potentials and predict redox spontaneity using standard electrode potentials The potential difference is caused by the ability of electrons to flow from one half cell to the other. describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths; in this explainer, we will learn how. Electrochemical Cells And Cell Potentials.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Electrochemical Cells And Cell Potentials electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Interpret electrode potentials in terms of relative oxidant and. Calculate cell potentials and predict redox spontaneity using standard electrode potentials Electrons are able to move between electrodes because the chemical reaction is a redox reaction. The potential difference is caused by the ability. Electrochemical Cells And Cell Potentials.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials; electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Interpret electrode potentials in terms of relative oxidant and reductant strengths; Electrons are able to move between electrodes because the chemical reaction is a redox reaction. describe and relate the definitions. Electrochemical Cells And Cell Potentials.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Electrochemical Cells And Cell Potentials electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Interpret electrode potentials in terms of relative oxidant and reductant strengths; the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Calculate cell potentials and predict redox spontaneity using standard electrode potentials Interpret. Electrochemical Cells And Cell Potentials.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Electrochemical Cells And Cell Potentials Interpret electrode potentials in terms of relative oxidant and reductant strengths; in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Calculate cell potentials and predict redox spontaneity using standard. Electrochemical Cells And Cell Potentials.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Electrochemical Cells And Cell Potentials Electrons are able to move between electrodes because the chemical reaction is a redox reaction. describe and relate the definitions of electrode and cell potentials; Interpret electrode potentials in terms of relative oxidant and reductant strengths; describe and relate the definitions of electrode and cell potentials; Interpret electrode potentials in terms of relative oxidant and reductant strengths; . Electrochemical Cells And Cell Potentials.
From www.studypool.com
SOLUTION CHEM151 Phoenix Electrochemical Cells and Cell Potentials Lab Electrochemical Cells And Cell Potentials electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Calculate cell potentials and predict redox spontaneity using standard electrode potentials The potential difference is caused by the ability of electrons to flow from one half cell to the other. describe and relate the definitions of electrode and cell potentials; Interpret electrode. Electrochemical Cells And Cell Potentials.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths; Electrons are able to move between electrodes because the chemical reaction is a redox reaction. Calculate cell potentials and predict redox spontaneity using standard electrode potentials describe and relate the definitions of electrode and cell potentials; The. Electrochemical Cells And Cell Potentials.
From www.scribd.com
Electrochemical Cells and Cell Potentials RPT PDF PDF Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and. Interpret electrode potentials in terms of relative oxidant and reductant strengths; Interpret electrode potentials in terms of relative oxidant and reductant strengths; describe and relate the definitions of electrode and cell potentials; electrochemical cells use redox reactions as. Electrochemical Cells And Cell Potentials.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID Electrochemical Cells And Cell Potentials electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Interpret electrode potentials in terms of relative oxidant and. Interpret electrode potentials in terms of relative oxidant and reductant strengths; The potential difference is caused by the ability of electrons to flow from one half cell to the other. describe and relate. Electrochemical Cells And Cell Potentials.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Electrochemical Cells And Cell Potentials Interpret electrode potentials in terms of relative oxidant and reductant strengths; electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. in this explainer, we will learn how to calculate the standard. Electrochemical Cells And Cell Potentials.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Electrochemical Cells And Cell Potentials Interpret electrode potentials in terms of relative oxidant and. describe and relate the definitions of electrode and cell potentials; in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. Electrons are able to move between electrodes because the chemical reaction is a redox reaction. Interpret electrode. Electrochemical Cells And Cell Potentials.
From mmerevise.co.uk
Electrochemical Cells MME Electrochemical Cells And Cell Potentials Interpret electrode potentials in terms of relative oxidant and reductant strengths; describe and relate the definitions of electrode and cell potentials; the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the. Electrochemical Cells And Cell Potentials.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and. describe and relate the definitions of electrode and cell potentials; describe and relate the definitions of electrode and cell potentials; Electrons are able to move between electrodes because the chemical reaction is a redox reaction. the cell. Electrochemical Cells And Cell Potentials.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Electrochemical Cells And Cell Potentials Calculate cell potentials and predict redox spontaneity using standard electrode potentials describe and relate the definitions of electrode and cell potentials; The potential difference is caused by the ability of electrons to flow from one half cell to the other. in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values. Electrochemical Cells And Cell Potentials.
From gaskatel.de
Principles of electrochemical cells Gaskatel Electrochemical Cells And Cell Potentials Interpret electrode potentials in terms of relative oxidant and reductant strengths; Interpret electrode potentials in terms of relative oxidant and. in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. Calculate cell potentials and predict redox spontaneity using standard electrode potentials Electrons are able to move between. Electrochemical Cells And Cell Potentials.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical Cells And Cell Potentials Interpret electrode potentials in terms of relative oxidant and reductant strengths; Interpret electrode potentials in terms of relative oxidant and reductant strengths; describe and relate the definitions of electrode and cell potentials; describe and relate the definitions of electrode and cell potentials; Electrons are able to move between electrodes because the chemical reaction is a redox reaction. The. Electrochemical Cells And Cell Potentials.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials; Interpret electrode potentials in terms of relative oxidant and reductant strengths; Calculate cell potentials and predict redox spontaneity using standard electrode potentials electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. describe and relate the definitions of electrode and cell. Electrochemical Cells And Cell Potentials.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials. the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant strengths; The potential difference is caused by the ability of electrons to flow from one half cell to the. Electrochemical Cells And Cell Potentials.
From usmle-rx.com
Cell Membrane Potential and Ion Balance USMLERx Electrochemical Cells And Cell Potentials Interpret electrode potentials in terms of relative oxidant and reductant strengths; The potential difference is caused by the ability of electrons to flow from one half cell to the other. Interpret electrode potentials in terms of relative oxidant and reductant strengths; Electrons are able to move between electrodes because the chemical reaction is a redox reaction. Interpret electrode potentials in. Electrochemical Cells And Cell Potentials.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Electrochemical Cells And Cell Potentials Calculate cell potentials and predict redox spontaneity using standard electrode potentials describe and relate the definitions of electrode and cell potentials; Interpret electrode potentials in terms of relative oxidant and reductant strengths; Interpret electrode potentials in terms of relative oxidant and reductant strengths; Interpret electrode potentials in terms of relative oxidant and. Calculate cell potentials and predict redox spontaneity. Electrochemical Cells And Cell Potentials.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemical Cells And Cell Potentials Calculate cell potentials and predict redox spontaneity using standard electrode potentials The potential difference is caused by the ability of electrons to flow from one half cell to the other. Interpret electrode potentials in terms of relative oxidant and reductant strengths; Interpret electrode potentials in terms of relative oxidant and. the cell potential, \(e_{cell}\), is the measure of the. Electrochemical Cells And Cell Potentials.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 Electrochemical Cells And Cell Potentials electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Interpret. Electrochemical Cells And Cell Potentials.
From learningschoolletopisaln.z22.web.core.windows.net
Schematic Of Electrochemical Cells Electrochemical Cells And Cell Potentials Interpret electrode potentials in terms of relative oxidant and. in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. Electrons are able to move between electrodes because the chemical reaction is a redox reaction. Interpret electrode potentials in terms of relative oxidant and reductant strengths; describe. Electrochemical Cells And Cell Potentials.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials. describe and relate the definitions of electrode and cell potentials; Interpret electrode potentials in terms of relative oxidant and reductant strengths; Calculate cell potentials and predict redox spontaneity using standard electrode potentials The potential difference is caused by the ability of electrons to flow from one half cell. Electrochemical Cells And Cell Potentials.
From enginediagrammerit.z21.web.core.windows.net
Electric Potential Cell Diagram Electrochemical Cells And Cell Potentials Interpret electrode potentials in terms of relative oxidant and. describe and relate the definitions of electrode and cell potentials; describe and relate the definitions of electrode and cell potentials; Electrons are able to move between electrodes because the chemical reaction is a redox reaction. Interpret electrode potentials in terms of relative oxidant and reductant strengths; the cell. Electrochemical Cells And Cell Potentials.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrochemical Cells And Cell Potentials describe and relate the definitions of electrode and cell potentials; The potential difference is caused by the ability of electrons to flow from one half cell to the other. in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. describe and relate the definitions of. Electrochemical Cells And Cell Potentials.
From www.studypool.com
SOLUTION Electrochemical Cells and Cell Potentials Discussion Studypool Electrochemical Cells And Cell Potentials the cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. describe and relate the definitions of electrode and cell potentials. The potential difference is caused by. Electrochemical Cells And Cell Potentials.
From www.slideserve.com
PPT Electrochemical Cells Voltage (Electric potential) PowerPoint Electrochemical Cells And Cell Potentials electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. describe and relate the definitions of electrode and cell potentials; describe and relate the definitions of electrode and cell potentials. Calculate cell potentials and predict redox spontaneity using standard electrode potentials Interpret electrode potentials in terms of relative oxidant and reductant. Electrochemical Cells And Cell Potentials.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Electrochemical Cells And Cell Potentials in this explainer, we will learn how to calculate the standard cell potential of galvanic cells using values from the electrochemical series. describe and relate the definitions of electrode and cell potentials; The potential difference is caused by the ability of electrons to flow from one half cell to the other. Interpret electrode potentials in terms of relative. Electrochemical Cells And Cell Potentials.