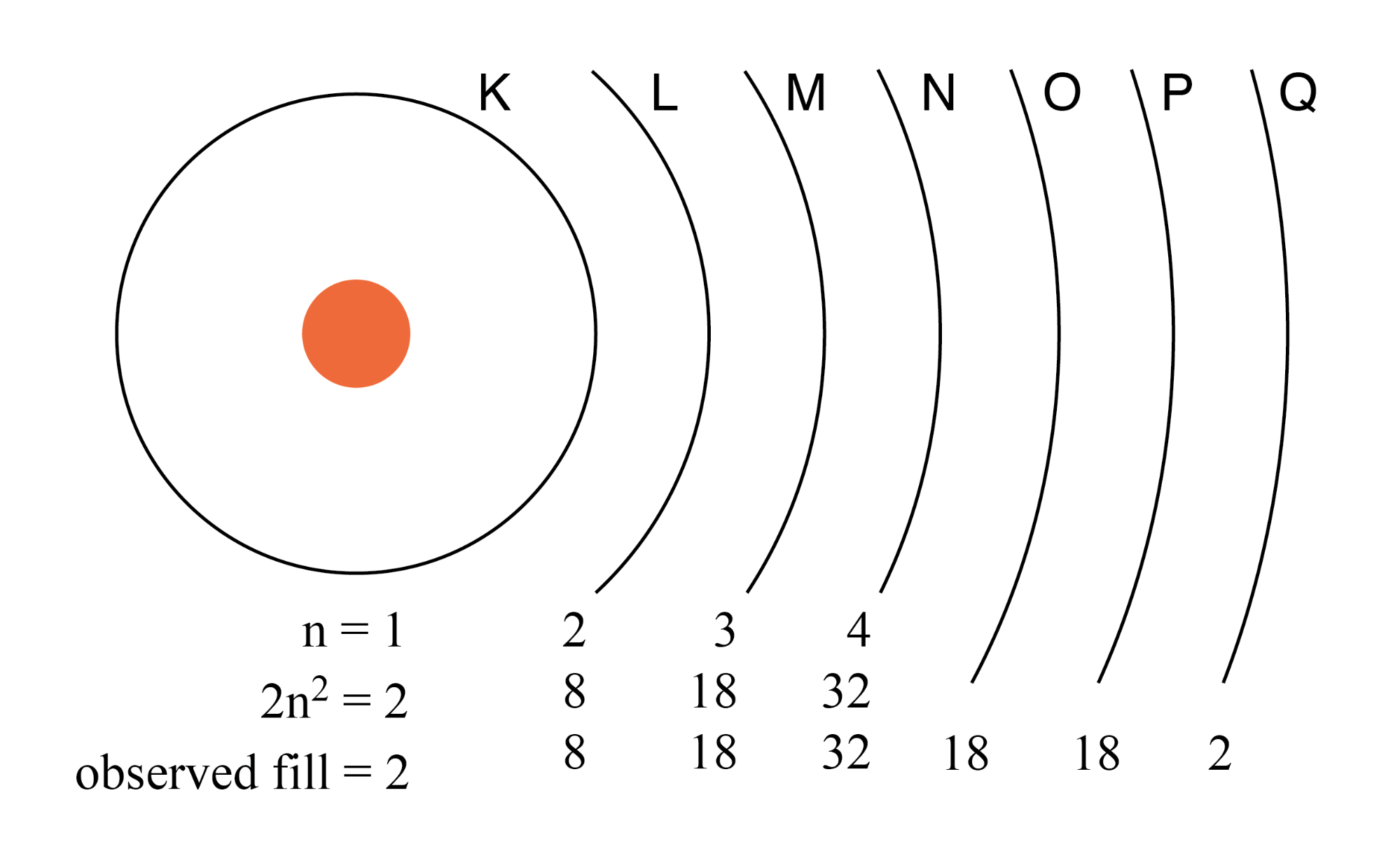
What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom . the first shell holds a maximum of 2 electrons in one 1s orbital; Calculate the maximum number of electrons that can occupy a shell with (a) n =. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; Orbitals that have the same or identical energy levels are referred to. this number indicates how many orbitals there are and thus how many electrons can reside in each atom. oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. The principal quantum number (n) cannot be zero. the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. The 3d, 4d etc., can. the three quantum numbers (n, l, and m) that describe an orbital are integers: The second shell holds a maximum of 8 electrons in one 2s and three. 0, 1, 2, 3, and so on. the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6).
from www.technocrazed.com
oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. Calculate the maximum number of electrons that can occupy a shell with (a) n =. the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). The principal quantum number (n) cannot be zero. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; 0, 1, 2, 3, and so on. the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. the three quantum numbers (n, l, and m) that describe an orbital are integers: The 3d, 4d etc., can. the first shell holds a maximum of 2 electrons in one 1s orbital;
2.2 Quantum Physics
What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the three quantum numbers (n, l, and m) that describe an orbital are integers: The 3d, 4d etc., can. the three quantum numbers (n, l, and m) that describe an orbital are integers: 0, 1, 2, 3, and so on. the first shell holds a maximum of 2 electrons in one 1s orbital; the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; Calculate the maximum number of electrons that can occupy a shell with (a) n =. Orbitals that have the same or identical energy levels are referred to. this number indicates how many orbitals there are and thus how many electrons can reside in each atom. The principal quantum number (n) cannot be zero. The second shell holds a maximum of 8 electrons in one 2s and three.
From www.chegg.com
Solved What is the maximum number of electrons that the What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom 0, 1, 2, 3, and so on. this number indicates how many orbitals there are and thus how many electrons can reside in each atom. the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). oxygen (atomic number 8) has a pair of electrons. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom The principal quantum number (n) cannot be zero. the three quantum numbers (n, l, and m) that describe an orbital are integers: Calculate the maximum number of electrons that can occupy a shell with (a) n =. the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. . What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From schematicdatacedars99.z22.web.core.windows.net
Electron Orbital Diagrams What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom this number indicates how many orbitals there are and thus how many electrons can reside in each atom. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; The principal quantum number (n) cannot be zero. the subshells s, p, d, and f contain. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom this number indicates how many orbitals there are and thus how many electrons can reside in each atom. the first shell holds a maximum of 2 electrons in one 1s orbital; The second shell holds a maximum of 8 electrons in one 2s and three. the subshells s, p, d, and f contain the following number of. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.thoughtco.com
Electron Configuration Chart What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). this number indicates how many orbitals there are and thus how many electrons can reside in each atom. The principal quantum number (n) cannot be zero. oxygen (atomic number 8) has a pair of. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.slideserve.com
PPT Sorbitals PowerPoint Presentation, free download ID1830175 What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). The 3d, 4d etc., can. 0, 1, 2, 3, and so on. the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. the three. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.youtube.com
12.1.4 State the maximum number of orbitals in a given energy level What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom this number indicates how many orbitals there are and thus how many electrons can reside in each atom. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; The principal quantum number (n) cannot be zero. 0, 1, 2, 3, and so on. The 3d,. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom this number indicates how many orbitals there are and thus how many electrons can reside in each atom. The 3d, 4d etc., can. the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. the first shell holds a maximum of 2 electrons in one 1s orbital; The. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID2664384 What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). The second shell holds a maximum of 8 electrons in one 2s and three. The 3d, 4d etc., can. this number indicates how many orbitals there are and thus how many electrons can reside in. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.technocrazed.com
2.2 Quantum Physics What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom The principal quantum number (n) cannot be zero. this number indicates how many orbitals there are and thus how many electrons can reside in each atom. 0, 1, 2, 3, and so on. Calculate the maximum number of electrons that can occupy a shell with (a) n =. The 3d, 4d etc., can. the 2p, 3p, 4p, etc.,. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the first shell holds a maximum of 2 electrons in one 1s orbital; the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.slideserve.com
PPT Electron Configurations and Orbital Diagrams PowerPoint What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). Orbitals that have the same or identical energy levels are referred to. the three quantum numbers (n, l, and m) that describe an orbital are integers: the general rule stating the number of electrons. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.nagwa.com
Question Video Identifying the Maximum Number of Electrons in the 1st What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom The second shell holds a maximum of 8 electrons in one 2s and three. The principal quantum number (n) cannot be zero. the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). The 3d, 4d etc., can. the first shell holds a maximum of 2. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom this number indicates how many orbitals there are and thus how many electrons can reside in each atom. Calculate the maximum number of electrons that can occupy a shell with (a) n =. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; Orbitals that. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From easamodules.blogspot.com
Physics For Foundation chapter 2matter (part 1) What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom The second shell holds a maximum of 8 electrons in one 2s and three. the first shell holds a maximum of 2 electrons in one 1s orbital; oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two.. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the three quantum numbers (n, l, and m) that describe an orbital are integers: Calculate the maximum number of electrons that can occupy a shell with (a) n =. The 3d, 4d etc., can. the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. The principal quantum number. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.youtube.com
What is the maximum number of orbitals that can be identified with the What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom The 3d, 4d etc., can. Orbitals that have the same or identical energy levels are referred to. the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). this number indicates how many orbitals there are and thus how many electrons can reside in each atom.. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.numerade.com
SOLVEDWhat is the maximum number of electrons in an atom that can have What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom 0, 1, 2, 3, and so on. this number indicates how many orbitals there are and thus how many electrons can reside in each atom. The principal quantum number (n) cannot be zero. oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From mavink.com
Electron Orbital Configuration Chart What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the first shell holds a maximum of 2 electrons in one 1s orbital; The 3d, 4d etc., can. Orbitals that have the same or identical energy levels are referred to. the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. the 2p, 3p, 4p, etc., can each. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.meta-synthesis.com
Quantum Number Periodic Table Chemogenesis What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom Calculate the maximum number of electrons that can occupy a shell with (a) n =. The second shell holds a maximum of 8 electrons in one 2s and three. oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.sciencefacts.net
Atomic Orbital Definition, Types, Shapes, and Diagram What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). Orbitals that have the same or identical energy levels are referred to. The principal quantum number (n) cannot be zero. The 3d, 4d etc., can. the subshells s, p, d, and f contain the following. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.slideserve.com
PPT Bohr’s model PowerPoint Presentation, free download ID2635508 What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. Calculate the maximum number of electrons that can occupy a shell with (a) n =. the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6).. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From vohobu-marria.blogspot.com
38 what is the maximum number of electrons that can occupy a box in an What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. the three quantum numbers (n, l, and m) that describe an orbital are integers: Calculate the maximum number of electrons that can occupy a shell with (a). What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From readingandwritingprojectcom.web.fc2.com
the maximum number of orbits in an atom is What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom The principal quantum number (n) cannot be zero. Calculate the maximum number of electrons that can occupy a shell with (a) n =. the three quantum numbers (n, l, and m) that describe an orbital are integers: the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. Orbitals. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the first shell holds a maximum of 2 electrons in one 1s orbital; The second shell holds a maximum of 8 electrons in one 2s and three. the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). this number indicates how many orbitals there. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.britannica.com
Orbital Chemistry, Physics & Applications Britannica What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom The 3d, 4d etc., can. the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. this number indicates how many orbitals there are and thus how many electrons can reside in each atom. Orbitals that have the same or identical energy levels are referred to. The principal quantum. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From chemistry.stackexchange.com
physical chemistry What are the maximum number of electrons in each What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom Orbitals that have the same or identical energy levels are referred to. this number indicates how many orbitals there are and thus how many electrons can reside in each atom. the first shell holds a maximum of 2 electrons in one 1s orbital; The 3d, 4d etc., can. The principal quantum number (n) cannot be zero. the. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From slideplayer.com
QUESTION What is the maximum number of of electrons that can be What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom this number indicates how many orbitals there are and thus how many electrons can reside in each atom. The 3d, 4d etc., can. The second shell holds a maximum of 8 electrons in one 2s and three. 0, 1, 2, 3, and so on. the three quantum numbers (n, l, and m) that describe an orbital are integers:. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.britannica.com
Basic concept and structure of an atom Britannica What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom Calculate the maximum number of electrons that can occupy a shell with (a) n =. The principal quantum number (n) cannot be zero. the 2p, 3p, 4p, etc., can each hold six electrons because they each have three orbitals, that can hold two electrons each (3*2=6). the subshells s, p, d, and f contain the following number of. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.numerade.com
SOLVEDWhat is the maximum number of electrons that can occupy the n=4 What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom this number indicates how many orbitals there are and thus how many electrons can reside in each atom. The 3d, 4d etc., can. Calculate the maximum number of electrons that can occupy a shell with (a) n =. the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up.. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From www.numerade.com
Consider the orbitals shown here in outline. (a) What is the maximum What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up. 0, 1, 2, 3, and so on. Calculate the maximum number of electrons that can occupy a shell with (a) n =. oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From courses.lumenlearning.com
Electrons Biology for Majors I What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; the three quantum numbers (n, l, and m) that describe an orbital are integers: 0, 1, 2, 3, and so on. The second shell holds a maximum of 8 electrons in one 2s and three.. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. the first shell holds a maximum of 2 electrons in one 1s orbital; the 2p, 3p, 4p, etc., can each hold six electrons because they each. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From circuitugandanur.z21.web.core.windows.net
Orbital Diagram Of Elements What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom The second shell holds a maximum of 8 electrons in one 2s and three. The 3d, 4d etc., can. oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. the general rule stating the number of electrons. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.
From schematiclistsuite99.z21.web.core.windows.net
Electron Orbital Diagrams What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom the first shell holds a maximum of 2 electrons in one 1s orbital; 0, 1, 2, 3, and so on. oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. The second shell holds a maximum of. What Is The Maximum Number Of Electrons In A Single 2P Orbital Of An Atom.