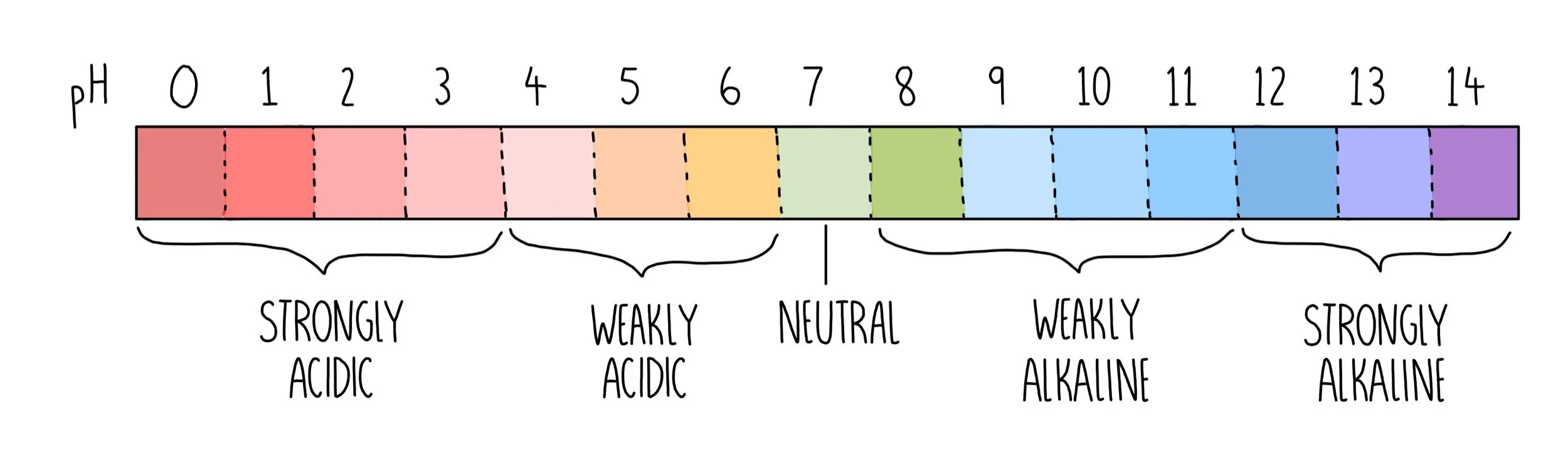
Indicator For Titration Weak Acid . The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. A ph curve is found if the ph of the solution being titrated is plotted against. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. The reaction of the weak acid, acetic acid, with a. Both indicators change colour over a specific. In this article, the author has explained acid base titration, its working principle. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Indicator & ph range table. The two most common indicators that are used in titrations are methyl orange and phenolphthalein.
from www.thesciencehive.co.uk
The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In this article, the author has explained acid base titration, its working principle. In this case, the weak acid. Both indicators change colour over a specific. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Indicator & ph range table. A ph curve is found if the ph of the solution being titrated is plotted against. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The reaction of the weak acid, acetic acid, with a. The two most common indicators that are used in titrations are methyl orange and phenolphthalein.
Acids, Alkalis and Titrations (GCSE) — the science hive
Indicator For Titration Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Both indicators change colour over a specific. In this article, the author has explained acid base titration, its working principle. The reaction of the weak acid, acetic acid, with a. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A ph curve is found if the ph of the solution being titrated is plotted against. Indicator & ph range table. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator For Titration Weak Acid Both indicators change colour over a specific. Indicator & ph range table. In this article, the author has explained acid base titration, its working principle. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The reaction of the weak acid, acetic acid, with a. The two most common indicators that are used in titrations are methyl. Indicator For Titration Weak Acid.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator For Titration Weak Acid The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In this article, the author has explained acid base titration, its working principle. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Indicator & ph range table. In this case,. Indicator For Titration Weak Acid.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator For Titration Weak Acid In this article, the author has explained acid base titration, its working principle. Indicator & ph range table. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The two most common indicators that are used. Indicator For Titration Weak Acid.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator For Titration Weak Acid A ph curve is found if the ph of the solution being titrated is plotted against. Indicator & ph range table. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The reaction of the weak acid, acetic acid, with a. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The. Indicator For Titration Weak Acid.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator For Titration Weak Acid A ph curve is found if the ph of the solution being titrated is plotted against. Indicator & ph range table. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In this case, the weak acid. The two most common indicators that are used in. Indicator For Titration Weak Acid.
From www.studypool.com
SOLUTION Acid base indicators titration curves Studypool Indicator For Titration Weak Acid Indicator & ph range table. In this article, the author has explained acid base titration, its working principle. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak. Indicator For Titration Weak Acid.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Indicator For Titration Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Indicator & ph range table. A ph curve is found if the ph of the solution being titrated is plotted against. The reaction of the weak acid, acetic acid, with a. In this article, the author. Indicator For Titration Weak Acid.
From mungfali.com
Acid Base Titration Indicator Indicator For Titration Weak Acid A ph curve is found if the ph of the solution being titrated is plotted against. Both indicators change colour over a specific. Indicator & ph range table. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. In this article, the author has explained acid base titration, its working principle. The titration of a. Indicator For Titration Weak Acid.
From slidetodoc.com
ACID BASE TITRATION INDICATORS Titration a method of Indicator For Titration Weak Acid Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this article, the author has explained acid base titration, its working principle. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a.. Indicator For Titration Weak Acid.
From wisc.pb.unizin.org
D30.1 AcidBase Indicators Chemistry 109 Fall 2021 Indicator For Titration Weak Acid A ph curve is found if the ph of the solution being titrated is plotted against. Indicator & ph range table. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. In this article, the author has explained acid base titration, its working principle. The two. Indicator For Titration Weak Acid.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Indicator For Titration Weak Acid The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In this article, the author has explained acid base titration, its working principle. A ph curve is found if the ph. Indicator For Titration Weak Acid.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Indicator For Titration Weak Acid Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The reaction of the weak acid, acetic acid, with a. Both indicators change colour over a specific. In this article, the author has explained acid base titration, its working principle. A ph. Indicator For Titration Weak Acid.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Indicator For Titration Weak Acid The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A ph curve is found if the ph of the solution being titrated is plotted against. In this article, the author has explained acid base titration, its working principle. Both indicators change colour over a specific. The reaction of the weak acid, acetic acid, with. Indicator For Titration Weak Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator For Titration Weak Acid A ph curve is found if the ph of the solution being titrated is plotted against. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. In this case, the weak acid. Both indicators change colour over a specific. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Indicator & ph. Indicator For Titration Weak Acid.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator For Titration Weak Acid The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. A ph curve is found if the ph of the solution being titrated is plotted against. The titration of a weak acid with a strong base involves the direct transfer. Indicator For Titration Weak Acid.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Indicator For Titration Weak Acid The reaction of the weak acid, acetic acid, with a. In this case, the weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Both indicators change colour over a specific. The two most common indicators that are used in titrations are methyl orange. Indicator For Titration Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Indicator For Titration Weak Acid Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. A ph curve is found if the ph of the solution being titrated is plotted against. Indicator & ph range table. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The titration of a weak acid with a strong base involves. Indicator For Titration Weak Acid.
From www.numerade.com
SOLVED Question 4 (1 point) The following graph is a titration curve Indicator For Titration Weak Acid In this article, the author has explained acid base titration, its working principle. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Both indicators change colour over a specific. The reaction of. Indicator For Titration Weak Acid.
From www.savemyexams.com
Choosing an AcidBase Indicator (HL) HL IB Chemistry Revision Notes 2025 Indicator For Titration Weak Acid The reaction of the weak acid, acetic acid, with a. In this case, the weak acid. Both indicators change colour over a specific. In this article, the author has explained acid base titration, its working principle. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. A ph curve is found if the ph of the solution. Indicator For Titration Weak Acid.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Indicator For Titration Weak Acid Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A ph curve is found if the ph of the solution being titrated is plotted against. The reaction of the weak acid, acetic acid, with a. Indicator & ph range table. The. Indicator For Titration Weak Acid.
From mmerevise.co.uk
pH Curves Questions and Revision MME Indicator For Titration Weak Acid In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Both indicators change colour over a specific. The reaction of the weak acid, acetic acid, with a. Indicator & ph range table. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common. Indicator For Titration Weak Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Indicator For Titration Weak Acid The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The reaction of the weak acid, acetic acid, with a. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Indicator & ph range table. Phenolphthalein is another commonly used indicator. Indicator For Titration Weak Acid.
From www.science-revision.co.uk
Titrations Indicator For Titration Weak Acid Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The reaction of the weak acid, acetic acid, with a. In this case, the weak acid. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The titration of a weak acid with a strong base involves the direct transfer of protons. Indicator For Titration Weak Acid.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Indicator For Titration Weak Acid The reaction of the weak acid, acetic acid, with a. Indicator & ph range table. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Both indicators change colour over a specific. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this article, the author has explained acid base titration,. Indicator For Titration Weak Acid.
From webmis.highland.cc.il.us
AcidBase Titrations Indicator For Titration Weak Acid The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The reaction of the weak acid, acetic acid, with a. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the.. Indicator For Titration Weak Acid.
From studylib.net
AcidBase Titrations Indicators Analytical Chemistry Indicator For Titration Weak Acid The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Both indicators change colour over a specific. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide. Indicator For Titration Weak Acid.
From mungfali.com
Acid Base Titration Indicator Indicator For Titration Weak Acid Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Both indicators change colour over a specific. Indicator & ph range table. In this case, the weak acid. A ph curve is found if the ph of the solution being titrated is plotted against. The reaction of the weak acid, acetic acid, with a. In this article,. Indicator For Titration Weak Acid.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Indicator For Titration Weak Acid Both indicators change colour over a specific. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In. Indicator For Titration Weak Acid.
From www.slideshare.net
Acid base titration Indicator For Titration Weak Acid Indicator & ph range table. The reaction of the weak acid, acetic acid, with a. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A ph curve is found if. Indicator For Titration Weak Acid.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Indicator For Titration Weak Acid In this case, the weak acid. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The reaction of the weak acid, acetic acid, with a. In this article, the author has explained acid base titration, its working principle. A ph curve is found if the ph of the solution being titrated is plotted against.. Indicator For Titration Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Indicator For Titration Weak Acid The reaction of the weak acid, acetic acid, with a. In this article, the author has explained acid base titration, its working principle. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Both indicators change colour over a specific. In this case, the weak acid. A ph curve is found if the ph of. Indicator For Titration Weak Acid.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Indicator For Titration Weak Acid Both indicators change colour over a specific. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The graph shows the results obtained. Indicator For Titration Weak Acid.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Indicator For Titration Weak Acid Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this article, the author has explained acid base titration, its working principle. In this case, the weak acid. Both indicators change colour over a specific. A ph curve is found if the ph of the solution being titrated is plotted against. The two most common indicators. Indicator For Titration Weak Acid.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Indicator For Titration Weak Acid Both indicators change colour over a specific. In this case, the weak acid. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The reaction of the weak acid, acetic acid, with a. A ph curve is found if the ph of the solution being titrated is plotted against. Indicator & ph range table. In. Indicator For Titration Weak Acid.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Indicator For Titration Weak Acid Both indicators change colour over a specific. The reaction of the weak acid, acetic acid, with a. In this article, the author has explained acid base titration, its working principle. A ph curve is found if the ph of the solution being titrated is plotted against. Indicator & ph range table. The two most common indicators that are used in. Indicator For Titration Weak Acid.