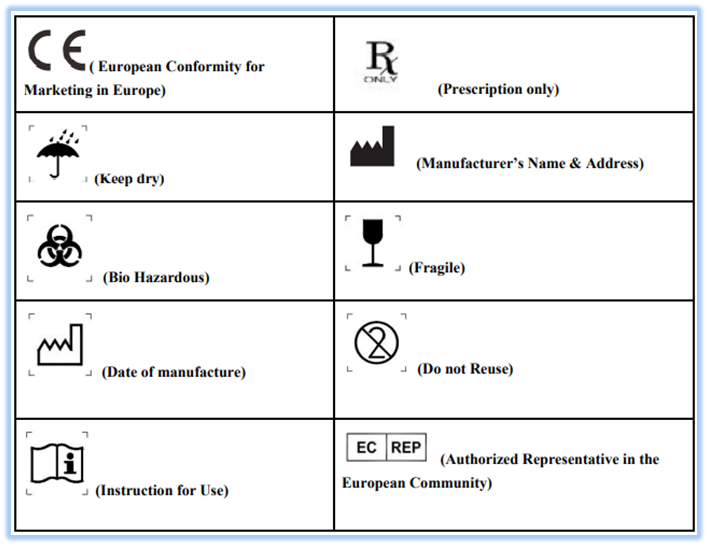
Medical Device Labeling Requirements Usa . (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. One thing the regulation makes. 21 cfr 801 covers general device labeling and the use of symbols. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Medical device labeling is a core requirement for getting your device onto the market. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr).
from knconsultingandservices.com
21 cfr 801 covers general device labeling and the use of symbols. One thing the regulation makes. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Medical device labeling is a core requirement for getting your device onto the market. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet.
What is Labelling? Medical Device Consulting Company
Medical Device Labeling Requirements Usa 21 cfr 801 covers general device labeling and the use of symbols. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Medical device labeling is a core requirement for getting your device onto the market. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. 21 cfr 801 covers general device labeling and the use of symbols. One thing the regulation makes.
From hiveta.com
Label Compliance AB&R® (American Barcode and RFID) Medical Device Labeling Requirements Usa Medical device labeling is a core requirement for getting your device onto the market. 21 cfr 801 covers general device labeling and the use of symbols. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Medical device manufacturers must incorporate. Medical Device Labeling Requirements Usa.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Medical Device Labeling Requirements Usa This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Labeling regulations pertaining to medical devices. Medical Device Labeling Requirements Usa.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Presentation ID8427354 Medical Device Labeling Requirements Usa (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. 21 cfr 801 covers general device labeling and the use of symbols. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical. Medical Device Labeling Requirements Usa.
From www.linkedin.com
Complying with Medical Device Labeling Requirements Medical Device Labeling Requirements Usa This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. One thing the regulation makes. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical device labeling is. Medical Device Labeling Requirements Usa.
From alysidia.com
21 CFR Part 801 FDA Labeling Requirements for Medical Devices Medical Device Labeling Requirements Usa Medical device labeling is a core requirement for getting your device onto the market. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. One thing the regulation makes. Labeling regulations pertaining to medical devices are found in the following parts. Medical Device Labeling Requirements Usa.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Registration & Labeling Medical Device Labeling Requirements Usa Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical device labeling is a core requirement for getting your device onto the market. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830. Medical Device Labeling Requirements Usa.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Presentation ID8427354 Medical Device Labeling Requirements Usa Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. (1) the label of every medical device shall bear a. Medical Device Labeling Requirements Usa.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Registration & Labeling Medical Device Labeling Requirements Usa Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. 21 cfr 801 covers general device labeling and the use of symbols. One thing the regulation makes. This post will discuss what counts as a medical device label, where they are required, and look at the key points of. Medical Device Labeling Requirements Usa.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Medical Device Labeling Requirements Usa This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. 21 cfr 801 covers general device. Medical Device Labeling Requirements Usa.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Medical Device Labeling Requirements Usa Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. One thing the regulation makes. 21 cfr 801 covers general device labeling and. Medical Device Labeling Requirements Usa.
From mungfali.com
FDA Medical Device Label Symbols Medical Device Labeling Requirements Usa Medical device labeling is a core requirement for getting your device onto the market. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. 21 cfr 801 covers general device labeling and the use of symbols. Labeling regulations pertaining to medical devices are found in. Medical Device Labeling Requirements Usa.
From www.youtube.com
U.S. FDA's Unique Device Identifier (UDI) Requirements YouTube Medical Device Labeling Requirements Usa (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This post will discuss what counts as a medical device label, where they. Medical Device Labeling Requirements Usa.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Presentation ID8427354 Medical Device Labeling Requirements Usa Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). 21 cfr 801 covers general device labeling and the use of symbols. Medical device labeling is a. Medical Device Labeling Requirements Usa.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Medical Device Labeling Requirements Usa Medical device labeling is a core requirement for getting your device onto the market. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. One thing the regulation makes. Medical device manufacturers must incorporate in their quality assurance (qa) program several. Medical Device Labeling Requirements Usa.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Labeling Requirements Usa One thing the regulation makes. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. 21 cfr 801 covers general device labeling and. Medical Device Labeling Requirements Usa.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Medical Device Labeling Requirements Usa Medical device labeling is a core requirement for getting your device onto the market. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. 21 cfr 801 covers general device labeling and the use of symbols. One thing the regulation makes. Labeling regulations pertaining to medical devices are found. Medical Device Labeling Requirements Usa.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Labeling Requirements Usa One thing the regulation makes. 21 cfr 801 covers general device labeling and the use of symbols. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. This post will discuss what counts as a medical device label, where they are required, and look at. Medical Device Labeling Requirements Usa.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Medical Device Labeling Requirements Usa Medical device labeling is a core requirement for getting your device onto the market. 21 cfr 801 covers general device labeling and the use of symbols. One thing the regulation makes. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations.. Medical Device Labeling Requirements Usa.
From ambitiousmares.blogspot.com
35 Medical Device Label Labels Design Ideas 2020 Medical Device Labeling Requirements Usa 21 cfr 801 covers general device labeling and the use of symbols. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. One thing the regulation makes. Labeling regulations pertaining to medical devices are found in the following parts of title. Medical Device Labeling Requirements Usa.
From www.tailoredlabel.com
UDI Label Requirements For FDA Medical Device Labels TLP Medical Device Labeling Requirements Usa Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. This post will discuss what counts as a medical device label, where they. Medical Device Labeling Requirements Usa.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Labeling Requirements Usa 21 cfr 801 covers general device labeling and the use of symbols. One thing the regulation makes. Medical device labeling is a core requirement for getting your device onto the market. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations.. Medical Device Labeling Requirements Usa.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Presentation ID8427354 Medical Device Labeling Requirements Usa Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical device labeling is a core requirement for getting your device onto the market. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830. Medical Device Labeling Requirements Usa.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Labeling Requirements Usa Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Medical device labeling is a core requirement for getting your device onto the market. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu. Medical Device Labeling Requirements Usa.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Medical Device Labeling Requirements Usa 21 cfr 801 covers general device labeling and the use of symbols. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). One thing the regulation makes. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. Medical Device Labeling Requirements Usa.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labeling Requirements Usa Medical device labeling is a core requirement for getting your device onto the market. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. One thing the regulation makes. Labeling regulations pertaining to medical devices are found in the following parts. Medical Device Labeling Requirements Usa.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Medical Device Labeling Requirements Usa 21 cfr 801 covers general device labeling and the use of symbols. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of. Medical Device Labeling Requirements Usa.
From www.scribd.com
Requirements For Labelling of Medical Devices Mda PDF Medical Device Packaging And Labeling Medical Device Labeling Requirements Usa One thing the regulation makes. Medical device labeling is a core requirement for getting your device onto the market. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. Medical Device Labeling Requirements Usa.
From www.slideshare.net
The basics of medical device labeling usa fda regulationsbycostas… Medical Device Labeling Requirements Usa 21 cfr 801 covers general device labeling and the use of symbols. Medical device labeling is a core requirement for getting your device onto the market. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This post will discuss what counts as a medical device label, where. Medical Device Labeling Requirements Usa.
From www.afpharmaservice.com
Medical Device Labelling Requirements Medical Device Labeling Requirements Usa One thing the regulation makes. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Medical device manufacturers must incorporate in their quality. Medical Device Labeling Requirements Usa.
From www.scribd.com
FDA Medical Device Labeling Requirements Checklist Greenlight Guru PDF Medical Device Labeling Requirements Usa 21 cfr 801 covers general device labeling and the use of symbols. Medical device labeling is a core requirement for getting your device onto the market. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. Labeling regulations pertaining to medical devices are found in the following parts of. Medical Device Labeling Requirements Usa.
From fra.animalia-life.club
Iso 15223 1 Symboles Medical Device Labeling Requirements Usa One thing the regulation makes. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). 21 cfr 801 covers general device labeling and. Medical Device Labeling Requirements Usa.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Medical Device Labeling Requirements Usa Medical device labeling is a core requirement for getting your device onto the market. Medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to labeling in order to meet. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of.. Medical Device Labeling Requirements Usa.
From www.pdffiller.com
Fillable Online GENERAL REQUIREMENTS & IMPORTANCE FOR LABELING OF MEDICAL DEVICES Fax Email Medical Device Labeling Requirements Usa (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. 21 cfr 801 covers general device. Medical Device Labeling Requirements Usa.
From www.schlafenderhase.com
Medical Device Labeling Requirements Schlafender Hase Medical Device Labeling Requirements Usa One thing the regulation makes. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical device labeling is. Medical Device Labeling Requirements Usa.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Medical Device Labeling Requirements Usa Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Medical device labeling is a core requirement for getting your device onto the market. One thing the regulation makes. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of. Medical Device Labeling Requirements Usa.