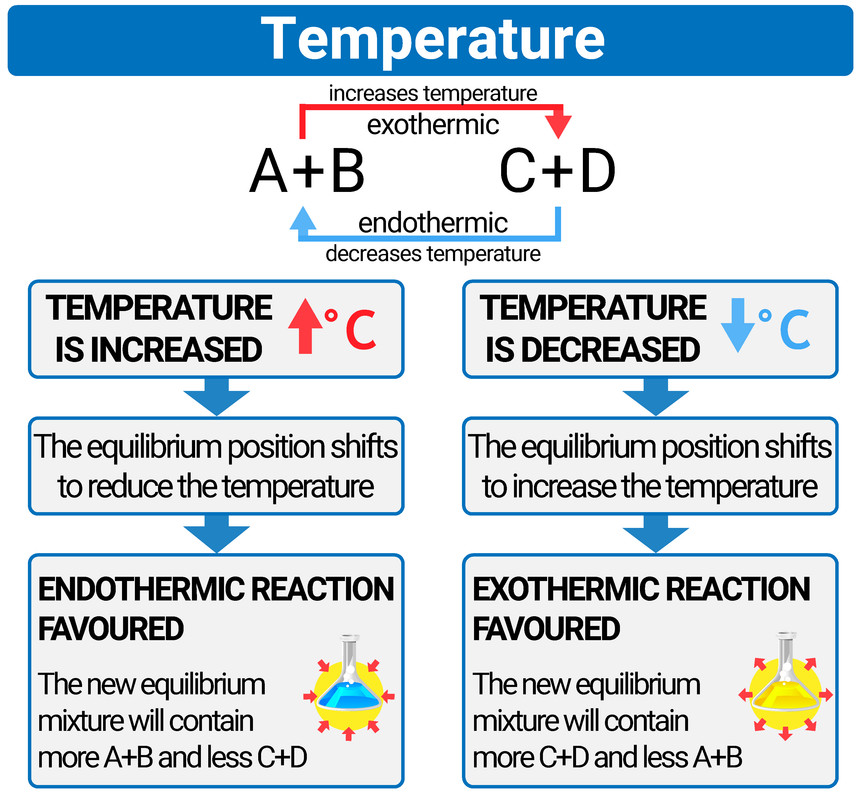
Does A Catalyst Affect Equilibrium . This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. However, a catalyst does not affect the extent or position of a reaction at. It does however allow equilibrium to be reached more. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates.
from revisechemistry.uk
As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. However, a catalyst does not affect the extent or position of a reaction at. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. It does however allow equilibrium to be reached more. The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium.
Dynamic Equilibria Edexcel T5 revisechemistry.uk
Does A Catalyst Affect Equilibrium Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. It does however allow equilibrium to be reached more. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. However, a catalyst does not affect the extent or position of a reaction at. As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Does A Catalyst Affect Equilibrium The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Adding a catalyst to a reaction at equilibrium has no effect on the position of. Does A Catalyst Affect Equilibrium.
From slideplayer.com
7 Energy, Rate, and Equilibrium GENERAL CHEMISTRY ppt download Does A Catalyst Affect Equilibrium The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. Overall, a catalyst is not a reactant and is not used up, but it still affects. Does A Catalyst Affect Equilibrium.
From www.slideserve.com
PPT Chapter 13 Chemical Equilibrium PowerPoint Presentation, free download ID4354049 Does A Catalyst Affect Equilibrium As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. However, a catalyst does not affect the extent or position of a reaction at. Because adding a catalyst doesn't affect the relative rates of. Does A Catalyst Affect Equilibrium.
From slideplayer.com
Types of Reactions and Activity Series ppt download Does A Catalyst Affect Equilibrium However, a catalyst does not affect the extent or position of a reaction at. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. It. Does A Catalyst Affect Equilibrium.
From slideplayer.com
Chemical Equilibrium ppt download Does A Catalyst Affect Equilibrium As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. It does however allow equilibrium to be reached more. A catalyst speeds up both the forward and the reverse. Does A Catalyst Affect Equilibrium.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.21C Understand Why a Catalyst Does Not Affect the Position of Does A Catalyst Affect Equilibrium However, a catalyst does not affect the extent or position of a reaction at. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Overall, a catalyst is not a. Does A Catalyst Affect Equilibrium.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free download ID6591070 Does A Catalyst Affect Equilibrium As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven. Does A Catalyst Affect Equilibrium.
From slideplayer.com
Chapter 14 Chemical Equilibrium ppt download Does A Catalyst Affect Equilibrium The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium.. Does A Catalyst Affect Equilibrium.
From www.slideshare.net
Intro to equilibrium abbrev alg Does A Catalyst Affect Equilibrium Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't. Does A Catalyst Affect Equilibrium.
From byjus.com
Why do catalyst not affect equilibrium? Does A Catalyst Affect Equilibrium The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. However, a catalyst does not affect the extent or position of a reaction at. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not. Does A Catalyst Affect Equilibrium.
From slideplayer.com
*Le Châtelier’s Principle and Equilibrium ppt download Does A Catalyst Affect Equilibrium Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. It does however allow equilibrium to be reached more. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Because adding a catalyst doesn't affect the relative rates of the two reactions, it. Does A Catalyst Affect Equilibrium.
From slideplayer.com
Factors That Affect Equilibrium ppt download Does A Catalyst Affect Equilibrium A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. It does however allow equilibrium to be reached more. This is because a catalyst speeds up the forward and back. Does A Catalyst Affect Equilibrium.
From h-o-m-e.org
Catalyst Tipping the Scales of Equilibrium Does A Catalyst Affect Equilibrium Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. It does however allow equilibrium to be reached more. As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. Overall, a catalyst is not a reactant and is not used. Does A Catalyst Affect Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID5967535 Does A Catalyst Affect Equilibrium It does however allow equilibrium to be reached more. However, a catalyst does not affect the extent or position of a reaction at. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. A catalyst speeds up both the forward and the reverse reactions, so there. Does A Catalyst Affect Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID6050390 Does A Catalyst Affect Equilibrium Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. However, a catalyst does not affect the extent or position of a reaction at. Adding a. Does A Catalyst Affect Equilibrium.
From slideplayer.com
Le Chatelier’s Principle and Equilibrium ppt download Does A Catalyst Affect Equilibrium However, a catalyst does not affect the extent or position of a reaction at. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of. Does A Catalyst Affect Equilibrium.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and reversible reactions Does A Catalyst Affect Equilibrium As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. Overall, a catalyst is. Does A Catalyst Affect Equilibrium.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Does A Catalyst Affect Equilibrium As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. This is because. Does A Catalyst Affect Equilibrium.
From byjus.com
3. How does catalysts affect The equilibrium? Does A Catalyst Affect Equilibrium It does however allow equilibrium to be reached more. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. Overall, a catalyst is not a reactant. Does A Catalyst Affect Equilibrium.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Does A Catalyst Affect Equilibrium The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium.. Does A Catalyst Affect Equilibrium.
From www.youtube.com
7.2.4 State and explain the effect of a catalyst on an equilibrium reaction. YouTube Does A Catalyst Affect Equilibrium Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. However, a catalyst does not affect the extent or position of a reaction at. The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. This is. Does A Catalyst Affect Equilibrium.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review PowerPoint Presentation ID6055094 Does A Catalyst Affect Equilibrium This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. However, a catalyst does not affect the extent or position of a reaction at. The presence. Does A Catalyst Affect Equilibrium.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation energy Ea of the overall Does A Catalyst Affect Equilibrium Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. It does however allow equilibrium to be reached more. Adding a catalyst to a reaction at equilibrium has no effect. Does A Catalyst Affect Equilibrium.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 Does A Catalyst Affect Equilibrium It does however allow equilibrium to be reached more. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. However, a catalyst does not affect the extent or position of a reaction at. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven. Does A Catalyst Affect Equilibrium.
From slideplayer.com
Chemical Equilibrium Chapter ppt download Does A Catalyst Affect Equilibrium This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. It does however allow equilibrium to be reached more. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Overall, a catalyst is not a reactant. Does A Catalyst Affect Equilibrium.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 Does A Catalyst Affect Equilibrium Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. However, a catalyst does. Does A Catalyst Affect Equilibrium.
From slideplayer.com
Equilibrium Chapter ppt download Does A Catalyst Affect Equilibrium This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. However, a catalyst does not affect the extent or position of a reaction at. It does however allow equilibrium to be reached more. Because adding a catalyst doesn't affect the relative rates of the two reactions,. Does A Catalyst Affect Equilibrium.
From revisechemistry.uk
Dynamic Equilibria Edexcel T5 revisechemistry.uk Does A Catalyst Affect Equilibrium Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a. Does A Catalyst Affect Equilibrium.
From www.savemyexams.com
The Position of Equilibrium Edexcel IGCSE Chemistry Revision Notes 2019 Does A Catalyst Affect Equilibrium A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. It does however allow equilibrium to be reached more. However, a catalyst does not affect the extent or position of. Does A Catalyst Affect Equilibrium.
From slideplayer.com
Chapter 14 Chemical Equilibrium ppt download Does A Catalyst Affect Equilibrium As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect. Does A Catalyst Affect Equilibrium.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Does A Catalyst Affect Equilibrium Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. It does however allow equilibrium to be reached more. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Overall, a catalyst is not a reactant and is not. Does A Catalyst Affect Equilibrium.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Chemistry Chemical Does A Catalyst Affect Equilibrium This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. However, a catalyst does not affect the extent or position of a reaction at. Because adding. Does A Catalyst Affect Equilibrium.
From www.slideserve.com
PPT Factors that Affect Equilibrium PowerPoint Presentation, free download ID3270711 Does A Catalyst Affect Equilibrium It does however allow equilibrium to be reached more. The presence of catalyst lowers the activation energies of both the forward and reverse reactions but does not affect the value of the equilibrium. However, a catalyst does not affect the extent or position of a reaction at. Adding a catalyst to a reaction at equilibrium has no effect on the. Does A Catalyst Affect Equilibrium.
From www.i-ciencias.com
físicoquímica ¿Que diagrama muestra el efecto de la Does A Catalyst Affect Equilibrium As we can see from the definition, a change in concentration (of the reactants/products), temperature, or pressure can shift the. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. Overall, a catalyst. Does A Catalyst Affect Equilibrium.
From www.numerade.com
⏩SOLVEDDoes a catalyst affect the value of the equilibrium… Numerade Does A Catalyst Affect Equilibrium Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. It does however. Does A Catalyst Affect Equilibrium.