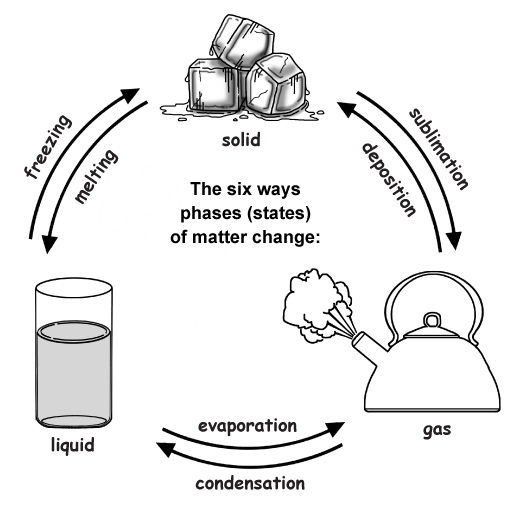
What's It Called When A Solid Goes To A Gas . The energy change required to. For any pure substance, the temperature at which. The opposite process, a liquid becoming a solid, is called solidification. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). This process is called deposition. It accounts for large losses of snow pack that never make it into a river, the routine. An example is the vaporization of frozen. The phase change from solid to gas is called sublimation. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid.
from www.exploringnature.org
Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. For any pure substance, the temperature at which. The phase change from solid to gas is called sublimation. An example is the vaporization of frozen. It accounts for large losses of snow pack that never make it into a river, the routine. The energy change required to. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called fusion (or melting). This process is called deposition.
Phases of Matter Gas, Liquids, Solids
What's It Called When A Solid Goes To A Gas Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. It accounts for large losses of snow pack that never make it into a river, the routine. For any pure substance, the temperature at which. An example is the vaporization of frozen. The conversion of a solid to a liquid is called fusion (or melting). Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The opposite process, a liquid becoming a solid, is called solidification. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy change required to. The phase change from solid to gas is called sublimation. This process is called deposition.
From theworldofmatterbymsk.weebly.com
SOLID, LIQUID & GAS THE WORLD OF MATTER What's It Called When A Solid Goes To A Gas Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. An example is the vaporization of frozen. It accounts for large losses of snow pack that never make it into a river, the routine. The energy change required to. The phase change from solid to gas is called sublimation. Sublimation, in physics, conversion of. What's It Called When A Solid Goes To A Gas.
From owlcation.com
All About Matter An Introduction to the Basics Owlcation What's It Called When A Solid Goes To A Gas The phase change from solid to gas is called sublimation. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). It accounts for large losses of snow pack that never make it into a river,. What's It Called When A Solid Goes To A Gas.
From www.toppr.com
Difference Between Solid Liquid and Gas with Definitions What's It Called When A Solid Goes To A Gas Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. The energy change required to. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called fusion (or melting). Sublimation, in physics, conversion of a substance from the. What's It Called When A Solid Goes To A Gas.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids What's It Called When A Solid Goes To A Gas Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. An example is the vaporization of frozen. The conversion of a solid to a liquid is called fusion (or melting). The energy change required to. It accounts for large losses of snow pack that never make it into a river, the routine. The energy. What's It Called When A Solid Goes To A Gas.
From www.snexplores.org
Explainer What are the different states of matter? What's It Called When A Solid Goes To A Gas The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). It accounts for large losses of snow pack that never make it into a river, the routine. The energy change required to. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The opposite process,. What's It Called When A Solid Goes To A Gas.
From www.thoughtco.com
List of Phase Changes Between States of Matter What's It Called When A Solid Goes To A Gas Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. It accounts for large losses of snow pack that never make it into a river, the routine. This process is called deposition. The energy change required to. An example is the vaporization of frozen. The opposite process, a liquid becoming a solid,. What's It Called When A Solid Goes To A Gas.
From socratic.org
What are examples of gases, liquids, and solids? Socratic What's It Called When A Solid Goes To A Gas It accounts for large losses of snow pack that never make it into a river, the routine. An example is the vaporization of frozen. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The energy change required to. The phase change from solid to gas is called sublimation. The energy required. What's It Called When A Solid Goes To A Gas.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Illustration What's It Called When A Solid Goes To A Gas The conversion of a solid to a liquid is called fusion (or melting). An example is the vaporization of frozen. The opposite process, a liquid becoming a solid, is called solidification. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Examples of gas to solid (deposition) under certain circumstances, gas can transform. What's It Called When A Solid Goes To A Gas.
From ar.inspiredpencil.com
Solid Liquid Gas Molecules What's It Called When A Solid Goes To A Gas An example is the vaporization of frozen. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). It accounts for large losses of snow pack that never make it into a river, the routine. For any pure. What's It Called When A Solid Goes To A Gas.
From www.slideserve.com
PPT Phas e Cha nges PowerPoint Presentation, free download ID6554404 What's It Called When A Solid Goes To A Gas An example is the vaporization of frozen. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). It accounts for large losses of snow pack that never make it into a river, the routine. The energy change required to. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into. What's It Called When A Solid Goes To A Gas.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID998654 What's It Called When A Solid Goes To A Gas The opposite process, a liquid becoming a solid, is called solidification. This process is called deposition. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The energy change required to. The conversion of a solid to a liquid is called fusion (or melting). Examples of gas to solid (deposition) under certain. What's It Called When A Solid Goes To A Gas.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry What's It Called When A Solid Goes To A Gas It accounts for large losses of snow pack that never make it into a river, the routine. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. For any pure substance, the temperature at which. The opposite process, a liquid becoming a solid, is called solidification. An example is the vaporization of. What's It Called When A Solid Goes To A Gas.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube What's It Called When A Solid Goes To A Gas Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The opposite process, a liquid becoming a solid, is called solidification. An example is the vaporization of frozen. It accounts for large losses of snow pack. What's It Called When A Solid Goes To A Gas.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas What's It Called When A Solid Goes To A Gas Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. It accounts for large losses of snow pack that never make it into a river, the routine. The conversion of a solid to a liquid is called fusion (or melting). The energy change required to. The energy required to melt 1 mol. What's It Called When A Solid Goes To A Gas.
From allaboutchemistry123.blogspot.com
What are solid, liquid, and gases? All About Chemistry What's It Called When A Solid Goes To A Gas This process is called deposition. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy change required to. For any pure substance,. What's It Called When A Solid Goes To A Gas.
From www.britannica.com
phase Definition & Facts Britannica What's It Called When A Solid Goes To A Gas The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). An example is the vaporization of frozen. The phase change from solid to gas is called sublimation. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. This process is called deposition. The conversion of a solid. What's It Called When A Solid Goes To A Gas.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to temperature. Vector Illustration What's It Called When A Solid Goes To A Gas Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. For any pure substance, the temperature at which. The opposite process, a liquid becoming a solid, is called solidification. An example is the vaporization of frozen. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The. What's It Called When A Solid Goes To A Gas.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram What's It Called When A Solid Goes To A Gas The energy change required to. An example is the vaporization of frozen. The opposite process, a liquid becoming a solid, is called solidification. It accounts for large losses of snow pack that never make it into a river, the routine. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. For any pure substance,. What's It Called When A Solid Goes To A Gas.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What's It Called When A Solid Goes To A Gas It accounts for large losses of snow pack that never make it into a river, the routine. For any pure substance, the temperature at which. The opposite process, a liquid becoming a solid, is called solidification. An example is the vaporization of frozen. The phase change from solid to gas is called sublimation. The energy change required to. The energy. What's It Called When A Solid Goes To A Gas.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk What's It Called When A Solid Goes To A Gas This process is called deposition. It accounts for large losses of snow pack that never make it into a river, the routine. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The opposite process, a liquid becoming a solid, is called solidification. The energy change required to. An example is the. What's It Called When A Solid Goes To A Gas.
From shaunmwilliams.com
Lecture 1 Presentation What's It Called When A Solid Goes To A Gas The opposite process, a liquid becoming a solid, is called solidification. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. The energy change required to. An example is the vaporization of frozen. It accounts for large losses of snow pack that never make it into a river, the routine. Sublimation, in physics, conversion. What's It Called When A Solid Goes To A Gas.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement and energy of the What's It Called When A Solid Goes To A Gas The conversion of a solid to a liquid is called fusion (or melting). For any pure substance, the temperature at which. This process is called deposition. An example is the vaporization of frozen. The energy change required to. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. Examples of gas to. What's It Called When A Solid Goes To A Gas.
From www.slideserve.com
PPT Chapter 1 Chemistry and Measurement PowerPoint Presentation, free download ID647250 What's It Called When A Solid Goes To A Gas This process is called deposition. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The phase change from solid to gas is called sublimation. An example is the vaporization of frozen. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. It accounts for large losses. What's It Called When A Solid Goes To A Gas.
From www.teachoo.com
Difference between Solid, Liquid, Gas in Table Form Teachoo What's It Called When A Solid Goes To A Gas The energy change required to. The phase change from solid to gas is called sublimation. For any pure substance, the temperature at which. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The conversion. What's It Called When A Solid Goes To A Gas.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii What's It Called When A Solid Goes To A Gas Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. This process is called deposition. The opposite process, a liquid becoming a solid, is called solidification. It accounts for large losses of snow pack that never make it into a river, the routine. For any pure substance, the temperature at which. The phase change. What's It Called When A Solid Goes To A Gas.
From www.youtube.com
Temperature and Solubility Solids and Gases YouTube What's It Called When A Solid Goes To A Gas Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The opposite process, a liquid becoming a solid, is called solidification. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy change required to. This process is called deposition. The conversion of a. What's It Called When A Solid Goes To A Gas.
From wirelistlatinised.z21.web.core.windows.net
Solid Liquid Gas Diagram What's It Called When A Solid Goes To A Gas The phase change from solid to gas is called sublimation. The conversion of a solid to a liquid is called fusion (or melting). It accounts for large losses of snow pack that never make it into a river, the routine. An example is the vaporization of frozen. This process is called deposition. The energy change required to. Sublimation, in physics,. What's It Called When A Solid Goes To A Gas.
From courses.lumenlearning.com
8.2 Solids and Liquids The Basics of General, Organic, and Biological Chemistry What's It Called When A Solid Goes To A Gas The opposite process, a liquid becoming a solid, is called solidification. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). It accounts for large losses of snow pack that never make it into a river, the routine. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a. What's It Called When A Solid Goes To A Gas.
From kids.britannica.com
solid Students Britannica Kids Homework Help What's It Called When A Solid Goes To A Gas The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). For any pure substance, the temperature at which. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. It accounts for large losses of snow pack that never make it into a river, the routine.. What's It Called When A Solid Goes To A Gas.
From www.istockphoto.com
Four States Of Matter Solid Liquid Gas And Plasma Stock Illustration Download Image Now iStock What's It Called When A Solid Goes To A Gas The energy change required to. It accounts for large losses of snow pack that never make it into a river, the routine. The phase change from solid to gas is called sublimation. An example is the vaporization of frozen. For any pure substance, the temperature at which. Sublimation, in physics, conversion of a substance from the solid to the gaseous. What's It Called When A Solid Goes To A Gas.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The F... Matter science What's It Called When A Solid Goes To A Gas The conversion of a solid to a liquid is called fusion (or melting). This process is called deposition. An example is the vaporization of frozen. Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. It accounts for large losses of snow pack that never make it into a river, the routine. The opposite. What's It Called When A Solid Goes To A Gas.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids What's It Called When A Solid Goes To A Gas It accounts for large losses of snow pack that never make it into a river, the routine. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The phase change from solid to gas is called sublimation. An example is the vaporization of frozen. For any pure substance, the temperature at which.. What's It Called When A Solid Goes To A Gas.
From www.ase.org.uk
Solids, liquids and gases What's It Called When A Solid Goes To A Gas The conversion of a solid to a liquid is called fusion (or melting). The opposite process, a liquid becoming a solid, is called solidification. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. This process is called deposition. The energy required to melt 1 mol of a substance is its enthalpy. What's It Called When A Solid Goes To A Gas.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What's It Called When A Solid Goes To A Gas The conversion of a solid to a liquid is called fusion (or melting). Examples of gas to solid (deposition) under certain circumstances, gas can transform directly into a solid. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The opposite process, a liquid becoming a solid, is called solidification. The phase change. What's It Called When A Solid Goes To A Gas.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps What's It Called When A Solid Goes To A Gas The opposite process, a liquid becoming a solid, is called solidification. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The phase change from solid to gas is called sublimation. It accounts for large losses of snow pack that never make it into a river, the routine. The energy required to. What's It Called When A Solid Goes To A Gas.