Medical Device Regulations Uae . A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. What are the conditions and requirements for obtaining a medical device? Are regulated by the ministry of health (moh) through the registration and drug control department. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. The ministry of health has issued registration. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. This article briefly outlines the regulatory approval process for medical devices in the uae. Marketing authorization holder companies and product.
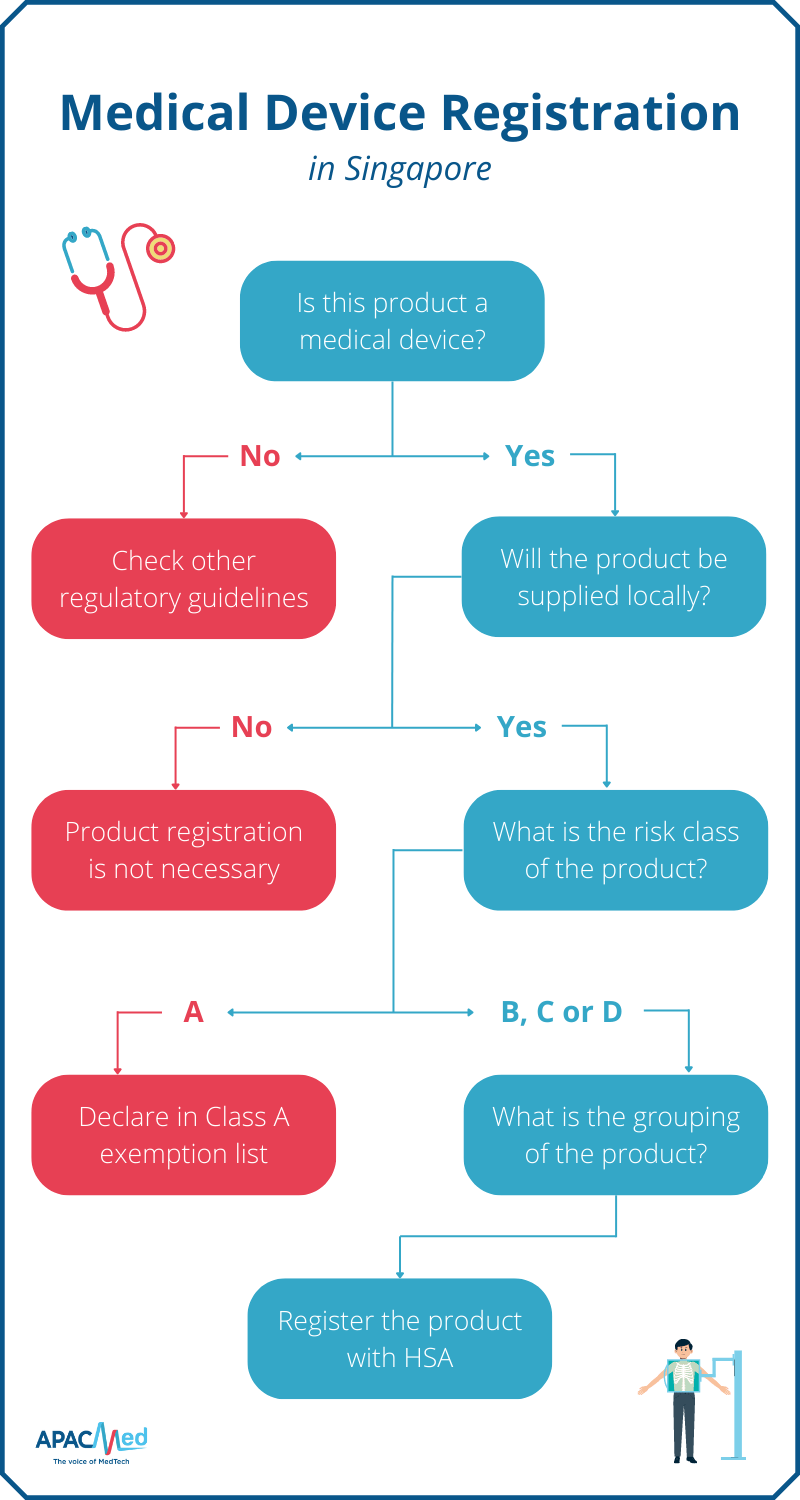
from apacmed.org
What are the conditions and requirements for obtaining a medical device? Marketing authorization holder companies and product. Are regulated by the ministry of health (moh) through the registration and drug control department. This article briefly outlines the regulatory approval process for medical devices in the uae. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. The ministry of health has issued registration. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse.
Medical Device Regulation Importance and Examples in APAC
Medical Device Regulations Uae This article briefly outlines the regulatory approval process for medical devices in the uae. Are regulated by the ministry of health (moh) through the registration and drug control department. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. This article briefly outlines the regulatory approval process for medical devices in the uae. The ministry of health has issued registration. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. Marketing authorization holder companies and product. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. What are the conditions and requirements for obtaining a medical device?
From familyclinic.netlify.app
Medical device regulations fda Medical Device Regulations Uae Marketing authorization holder companies and product. This article briefly outlines the regulatory approval process for medical devices in the uae. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. What are the conditions and requirements for obtaining a medical device? The ministry of health has issued registration. Provide laboratory. Medical Device Regulations Uae.
From medicaldevicehq.com
Medical Device Regulation codes Medical Device HQ 1 Medical Device Regulations Uae The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. This article briefly outlines the regulatory approval process for medical devices in the uae. 8 of 2019 on medical products, pharmacy profession and. Medical Device Regulations Uae.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Medical Device Regulations Uae What are the conditions and requirements for obtaining a medical device? Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. Are regulated by the ministry of health (moh) through the registration and drug control department. This article briefly. Medical Device Regulations Uae.
From readmagazine.com
Unlocking the Secrets of Medical Device Regulations A Comprehensive Medical Device Regulations Uae The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. This article briefly outlines the regulatory approval process for medical devices in the uae. What are the conditions and requirements for obtaining. Medical Device Regulations Uae.
From www.scribd.com
Medical device Regulations PDF Medical Device Verification And Medical Device Regulations Uae Are regulated by the ministry of health (moh) through the registration and drug control department. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. This article briefly outlines the regulatory approval process for medical devices in the uae. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law). Medical Device Regulations Uae.
From www.gcp-service.com
MDR and ISO14155 Compliant Quality Management Systems in Clinical Medical Device Regulations Uae The ministry of health has issued registration. Marketing authorization holder companies and product. What are the conditions and requirements for obtaining a medical device? 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. This article briefly outlines the regulatory approval process for medical devices in the uae. Are regulated by the. Medical Device Regulations Uae.
From gcpcentral.com
Medical Device Regulation The Need for Clinical Expert Involvement Medical Device Regulations Uae The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. Are regulated by the ministry of health (moh) through the. Medical Device Regulations Uae.
From readmagazine.com
Unlocking the Secrets of Medical Device Regulations A Comprehensive Medical Device Regulations Uae What are the conditions and requirements for obtaining a medical device? Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. The ministry of health has issued registration. This article briefly outlines the regulatory approval process for medical devices in the uae. Marketing authorization holder companies and product. 8 of 2019 on medical products, pharmacy profession and. Medical Device Regulations Uae.
From www.youtube.com
Understanding Medical Device Regulations YouTube Medical Device Regulations Uae 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. The ministry of health has issued registration. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. This article briefly outlines the regulatory approval process for medical devices in the uae. What are the conditions and requirements for obtaining. Medical Device Regulations Uae.
From globalpccs.com
EU Medical Device Regulation Compliance Services in IMDS CDX ELV Medical Device Regulations Uae The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. Are regulated by the ministry of health (moh) through the registration and drug control department. Marketing authorization holder companies and product. A medical product that contains a. Medical Device Regulations Uae.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Regulations Uae The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. What are the conditions and requirements for obtaining a medical device? 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. Marketing authorization holder companies and product. Are regulated by the ministry. Medical Device Regulations Uae.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Medical Device Regulations Uae This article briefly outlines the regulatory approval process for medical devices in the uae. Are regulated by the ministry of health (moh) through the registration and drug control department. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law). Medical Device Regulations Uae.
From www.dreamstime.com
Medical Device Regulation MDR is Shown Using the Text Stock Photo Medical Device Regulations Uae The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. The ministry of health has issued registration. This article briefly outlines the regulatory approval process for medical devices in the uae. Are regulated by the ministry of health (moh) through the registration and drug control department. 8 of 2019 on. Medical Device Regulations Uae.
From alirahealth.com
Medical Device Regulation (MDR) Support Alira Health Medical Device Regulations Uae Marketing authorization holder companies and product. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. What are the conditions and requirements for obtaining a medical device? The ministry of health has issued registration. 8 of 2019. Medical Device Regulations Uae.
From www.rizmona.com
Medical Device Registration in the UAE Riz & Mona Medical Device Regulations Uae Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. A medical product that contains a substance, device, tool,. Medical Device Regulations Uae.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Medical Device Regulations Uae 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. What are the conditions and requirements for obtaining a medical device? Are regulated by the ministry of health (moh) through the registration. Medical Device Regulations Uae.
From www.slideshare.net
Global medical device regulations Medical Device Regulations Uae The ministry of health has issued registration. This article briefly outlines the regulatory approval process for medical devices in the uae. Marketing authorization holder companies and product. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. What are the. Medical Device Regulations Uae.
From productregistrationdubai.com
Medical Device Registration in Dubai UAE Register Health Items PRD Medical Device Regulations Uae Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. Marketing authorization holder companies and product. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. What are the conditions and requirements for obtaining a medical device? 8 of 2019 on medical products, pharmacy profession and pharmaceutical. Medical Device Regulations Uae.
From kladuvsja.blob.core.windows.net
Medical Device Regulation Eu at Hay blog Medical Device Regulations Uae Marketing authorization holder companies and product. What are the conditions and requirements for obtaining a medical device? Are regulated by the ministry of health (moh) through the registration and drug control department. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. The ministry of health has issued registration. Provide. Medical Device Regulations Uae.
From blog.cosmotrace.com
Medical Devices Regulations (MDR) Medical Device Regulations Uae What are the conditions and requirements for obtaining a medical device? Marketing authorization holder companies and product. Are regulated by the ministry of health (moh) through the registration and drug control department. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. This article briefly outlines the regulatory approval process for medical. Medical Device Regulations Uae.
From www.eclevarmedtech.com
A Guide to Medical Devices Regulations Everything You Need to Know Medical Device Regulations Uae This article briefly outlines the regulatory approval process for medical devices in the uae. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. Are regulated by the ministry of health (moh) through. Medical Device Regulations Uae.
From www.slideshare.net
Infographics 9 healthcare regulations uae Medical Device Regulations Uae The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. This article briefly outlines the regulatory approval process for medical devices in the uae. A medical product that contains a substance, device,. Medical Device Regulations Uae.
From gxp-training.com
Medical Device Regulation MDR 2017/745 Course and Certificate Medical Device Regulations Uae 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. Are regulated by the ministry of health (moh) through the registration and drug control department. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. What are the conditions and requirements for obtaining a medical device? The ministry of. Medical Device Regulations Uae.
From security.cybellum.com
Intro to Medical Device Standards & Regulations Cybellum Medical Device Regulations Uae Are regulated by the ministry of health (moh) through the registration and drug control department. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. A medical product that contains a substance, device, tool, implant, instrument, detector or system,. Medical Device Regulations Uae.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Medical Device Regulations Uae Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. The ministry of health has issued registration. This article briefly outlines the regulatory approval process for medical devices in the uae. 8 of 2019 on medical products, pharmacy profession and. Medical Device Regulations Uae.
From crfweb.com
Medical Device Regulations Medical Device Regulations Uae A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. Are regulated by the ministry of health (moh) through the registration and drug control department. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. This article briefly outlines the regulatory approval process for medical. Medical Device Regulations Uae.
From www.linkedin.com
AI Regulatory Frameworks for Medical Devices Harmonization vs Local Medical Device Regulations Uae This article briefly outlines the regulatory approval process for medical devices in the uae. The ministry of health has issued registration. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. What are the conditions and requirements for obtaining a medical device? 8 of 2019 on medical products, pharmacy profession. Medical Device Regulations Uae.
From www.protoexpress.com
Medical Device Regulations for PCBA Sierra Circuits Medical Device Regulations Uae The ministry of health has issued registration. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. This article briefly outlines the regulatory approval process for medical devices in the uae. Are regulated by the ministry of health (moh) through the registration and drug control department. Marketing authorization holder companies. Medical Device Regulations Uae.
From www.slideserve.com
PPT Global Medical Device Regulations PowerPoint Presentation, free Medical Device Regulations Uae 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. What are the conditions and requirements for obtaining a medical device? This article briefly outlines the regulatory approval process for medical devices in the uae. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories.. Medical Device Regulations Uae.
From www.tuvsud.com
Infographic The Medical Device Regulation TÜV SÜD Medical Device Regulations Uae Marketing authorization holder companies and product. The ministry of health has issued registration. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. Are regulated by the ministry of health (moh) through the registration and drug control department. A medical product that contains a substance, device, tool, implant, instrument, detector. Medical Device Regulations Uae.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Regulations Uae Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. What are the conditions and requirements for obtaining a medical device? A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. The. Medical Device Regulations Uae.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulations Uae A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. Are regulated by the ministry of health (moh) through the registration and drug control department. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. The purpose of this standard is to set the requirements of reporting medical device problems,. Medical Device Regulations Uae.
From www.medicaldevice-network.com
Medical Devices Regulation 2020 (MDR) to improve industry standards. Medical Device Regulations Uae A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. Marketing authorization holder companies and product. What are the conditions and requirements. Medical Device Regulations Uae.
From medicaldevicehq.com
MDR Article 22 Medical Device HQ Medical Device Regulations Uae 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. The purpose of this standard is to set the requirements of reporting medical device problems, medical device incidents & adverse. Are regulated by the ministry of health (moh) through the registration and drug control department. Provide laboratory requirements and analysis, as well. Medical Device Regulations Uae.
From www.regdesk.co
EMA Guidance on Medicines Used in Medical Devices RegDesk Medical Device Regulations Uae Provide laboratory requirements and analysis, as well as pricing for certain medical equipment. 8 of 2019 on medical products, pharmacy profession and pharmaceutical establishments (the new law) aims to consolidate and. A medical product that contains a substance, device, tool, implant, instrument, detector or system, including its accessories. Marketing authorization holder companies and product. The purpose of this standard is. Medical Device Regulations Uae.