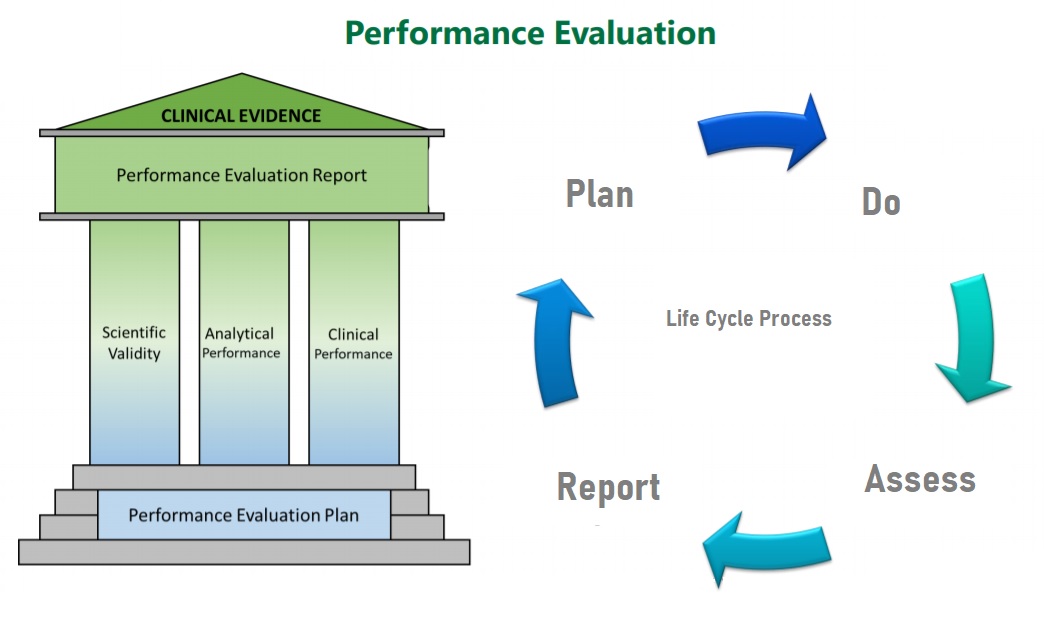
Performance Evaluation Report Template Ivdr . The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. Pers consist of three pillars: Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one of those is the need for a. The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd.
from www.clinicalevaluation-report.com
Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one of those is the need for a. The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. Pers consist of three pillars: Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd.
IVDR Performance Evaluation Report Template
Performance Evaluation Report Template Ivdr Pers consist of three pillars: Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one of those is the need for a. The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Pers consist of three pillars: Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible.
From www.orielstat.com
PER (Performance Evaluation Report) Training for EU IVDR Compliance Performance Evaluation Report Template Ivdr Pers consist of three pillars: Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. Under the eu. Performance Evaluation Report Template Ivdr.
From www.rqmplus.com
The IVDR Performance Evaluation Report Performance Evaluation Report Template Ivdr This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. Pers consist of three pillars:. Performance Evaluation Report Template Ivdr.
From www.clinicalevaluation-report.com
IVDR Performance Evaluation Report Template Performance Evaluation Report Template Ivdr The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Performance evaluation is defined in. Performance Evaluation Report Template Ivdr.
From www.slideshare.net
Performance evaluation report template PDF Performance Evaluation Report Template Ivdr Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. Pers consist of three pillars: The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one of those is the need for a. Find out how the. Performance Evaluation Report Template Ivdr.
From www.template.net
Performance Evaluation Template Google Docs, Word, Apple Pages, PDF Performance Evaluation Report Template Ivdr Pers consist of three pillars: Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). The performance evaluation report (per) is the culmination of the application. Performance Evaluation Report Template Ivdr.
From www.template.net
FREE Evaluation Report Templates Download in Word, Google Docs, PDF Performance Evaluation Report Template Ivdr Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one of. Performance Evaluation Report Template Ivdr.
From criterionedge.com
IVDR Performance Evaluation Report Guidance From An Expert Performance Evaluation Report Template Ivdr The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. Pers consist of three pillars: This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of. Performance Evaluation Report Template Ivdr.
From www.slideserve.com
PPT EU IVDR Performance Validation Report Steps and Requirements Performance Evaluation Report Template Ivdr The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify. Performance Evaluation Report Template Ivdr.
From templatelab.com
46 Employee Evaluation Forms & Performance Review Examples Performance Evaluation Report Template Ivdr Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). Pers consist of three pillars: The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one of those is the need for a. Find out how the requirements of the ivdr. Performance Evaluation Report Template Ivdr.
From www.sampletemplates.com
Evaluation Report Template 10+ Free Samples, Examples, Format Performance Evaluation Report Template Ivdr Pers consist of three pillars: The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. The performance evaluation report (per) is the culmination of the application of. Performance Evaluation Report Template Ivdr.
From www.sampletemplates.com
12+ Sample Evaluation Reports Sample Templates Performance Evaluation Report Template Ivdr The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to. Performance Evaluation Report Template Ivdr.
From www.orielstat.com
IVD Manufacturer Clinical Evidence Needed for IVDR Performance Performance Evaluation Report Template Ivdr Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. The ivdr (eu 2017/746) brings new requirements for. Performance Evaluation Report Template Ivdr.
From www.celegence.com
PMS & Performance Evaluation under the IVDR inar Replay Performance Evaluation Report Template Ivdr The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as. Performance Evaluation Report Template Ivdr.
From www.sampletemplates.com
10 Job Performance Evaluation Templates Download for Free Sample Performance Evaluation Report Template Ivdr Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). Pers consist of three pillars: The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify. Performance Evaluation Report Template Ivdr.
From clin-r.com
IVD Performance Evaluation Planning & Report (PER) Clin R Performance Evaluation Report Template Ivdr The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make. Performance Evaluation Report Template Ivdr.
From www.greenlight.guru
Performance Evaluation Under EU IVDR Proving Your IVD’s Performance Performance Evaluation Report Template Ivdr Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. Pers consist of three pillars: Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). The ivdr (eu 2017/746) brings new requirements. Performance Evaluation Report Template Ivdr.
From www.sampletemplates.com
FREE 6+ Sample Performance Evaluation Forms in PDF MS Word Performance Evaluation Report Template Ivdr Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. Pers consist of three pillars: Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of. Performance Evaluation Report Template Ivdr.
From venngage.com
Employee Performance Assessment Report Template Venngage Performance Evaluation Report Template Ivdr Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. This document outlines the general principles of clinical evidence and provides guidance on the continuous process. Performance Evaluation Report Template Ivdr.
From www.youtube.com
IVDR Essentials Performance Evaluation Report Early Planning and Performance Evaluation Report Template Ivdr Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). Pers consist of three pillars: The ivdr (eu 2017/746) brings new requirements. Performance Evaluation Report Template Ivdr.
From www.youtube.com
Mastering the challenges In vitro Diagnostics Directive (IVDR) and Performance Evaluation Report Template Ivdr This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one. Performance Evaluation Report Template Ivdr.
From www.degruyter.com
Clinical evidence requirements according to the IVDR 2017/746 Performance Evaluation Report Template Ivdr Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. This document outlines the general principles of clinical. Performance Evaluation Report Template Ivdr.
From www.orielstat.com
Understanding EU IVD Performance Evaluation Plan and Report Performance Evaluation Report Template Ivdr This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to. Performance Evaluation Report Template Ivdr.
From www.sampletemplates.com
FREE 8+ Sample Job Evaluation Reports in MS Word PDF Performance Evaluation Report Template Ivdr This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. Pers consist of three pillars: Find out how the requirements of the ivdr for the performance evaluation differ from those. Performance Evaluation Report Template Ivdr.
From www.reportexamples.org
Performance Evaluation Report Sample → Free Report Examples Performance Evaluation Report Template Ivdr This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one. Performance Evaluation Report Template Ivdr.
From www.formsbank.com
Fillable Performance Evaluation Report printable pdf download Performance Evaluation Report Template Ivdr This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. Pers consist of three pillars: The performance evaluation report contains the methods. Performance Evaluation Report Template Ivdr.
From www.celegence.com
Ensuring Compliance for your IVD's Performance Evaluation Part 1 Performance Evaluation Report Template Ivdr Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. Pers consist of three pillars: Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). This document outlines the general principles of clinical evidence and provides. Performance Evaluation Report Template Ivdr.
From compliancenavigator.bsigroup.com
IVDR Practical Considerations for the Performance Evaluation Plan and Performance Evaluation Report Template Ivdr The performance evaluation report (per) is the culmination of the application of the performance evaluation process to a specific ivd. This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify. Performance Evaluation Report Template Ivdr.
From clin-r.com
IVD Performance Evaluation Planning & Report (PER) Clin R Performance Evaluation Report Template Ivdr This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. The performance evaluation report contains the methods and results regarding scientific validity, analytical performance and. Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). Performance evaluation is defined in. Performance Evaluation Report Template Ivdr.
From easymedicaldevice.com
GSPR General Safety And Performance Requirements [EU MDR & IVDR] Performance Evaluation Report Template Ivdr The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one of those is the need for a. Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). Performance evaluation is defined in article 2 of ivdr as ‘an assessment and. Performance Evaluation Report Template Ivdr.
From template.mammycares.com
Performance Review Template Word Free Free Printable Template Performance Evaluation Report Template Ivdr Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). Pers consist of three pillars: Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. The performance evaluation report (per) is the culmination of the application. Performance Evaluation Report Template Ivdr.
From www.distillersr.com
How Literature Review Automation Improves CER and PER Program Performance Evaluation Report Template Ivdr This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. Under the eu in vitro diagnostic medical devices regulation 2017/746 (ivdr), every ivd must have a performance evaluation report (per). The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one. Performance Evaluation Report Template Ivdr.
From www.slideteam.net
Top 10 Employee Review Report Templates with Samples and Examples Performance Evaluation Report Template Ivdr This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. The ivdr (eu 2017/746) brings new requirements for manufacturers with regard to performance evaluation and clinical performance studies and one of those is the need for a. Pers consist of three pillars: The performance evaluation report contains the methods and. Performance Evaluation Report Template Ivdr.
From www.templateral.com
√ Free Printable Employee Performance Evaluation Template Performance Evaluation Report Template Ivdr Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. This document outlines the general principles of clinical. Performance Evaluation Report Template Ivdr.
From edit.org
Free Employee Performance Review Templates Performance Evaluation Report Template Ivdr Performance evaluation is defined in article 2 of ivdr as ‘an assessment and analysis of data to establish or verify the scientific validity, the. Pers consist of three pillars: Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. The performance evaluation. Performance Evaluation Report Template Ivdr.
From www.sampletemplates.com
FREE 9+ Sample Performance Evaluation Templates in PDF MS Word Performance Evaluation Report Template Ivdr Pers consist of three pillars: Find out how the requirements of the ivdr for the performance evaluation differ from those of the ivdd to make sure you achieve conformity as quickly as possible. This document outlines the general principles of clinical evidence and provides guidance on the continuous process of performance evaluation for. The performance evaluation report (per) is the. Performance Evaluation Report Template Ivdr.