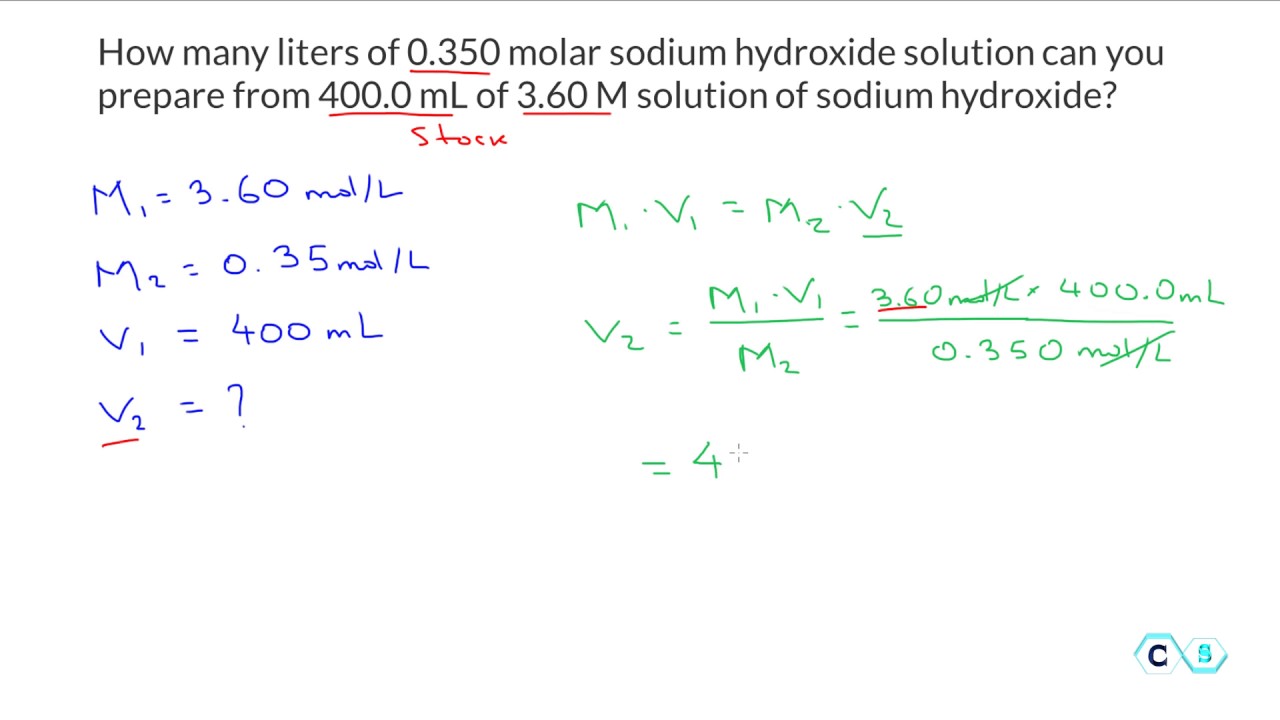
Molarity Dilution Worksheet . Find the molarity (concentration) of the following solutions: Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. Calculate the molarity of a solution containing 10.0 grams of sulfuric. Chemistry ii worksheet molarity, & dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 2) if i add water to 100 ml of a 0.15. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. Volume must be in liters! Work each of the following problems in the space provided.
from materialmediaunchain.z5.web.core.windows.net
Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. Find the molarity (concentration) of the following solutions: 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. Volume must be in liters! Chemistry ii worksheet molarity, & dilution. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. 2) if i add water to 100 ml of a 0.15. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Work each of the following problems in the space provided.
Molarity And Dilution Worksheet
Molarity Dilution Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 2) if i add water to 100 ml of a 0.15. Volume must be in liters! 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. Find the molarity (concentration) of the following solutions: Work each of the following problems in the space provided. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Chemistry ii worksheet molarity, & dilution. Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. Calculate the molarity of a solution containing 10.0 grams of sulfuric.
From worksheets.clipart-library.com
Free molarity calculations worksheet, Download Free molarity Molarity Dilution Worksheet Work each of the following problems in the space provided. Find the molarity (concentration) of the following solutions: Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Calculate the molarity of a solution containing 10.0 grams of sulfuric. 1) if i add 25 ml of water to 125 ml. Molarity Dilution Worksheet.
From www.onlineworksheet.my.id
Molarity Practice Worksheet Answer Molarity Dilution Worksheet Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. Find the molarity (concentration) of the following solutions: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. Molarity Dilution Worksheet.
From davida.davivienda.com
Molarity Dilution Worksheet Printable Word Searches Molarity Dilution Worksheet Calculate the molarity of a solution containing 10.0 grams of sulfuric. Volume must be in liters! Chemistry ii worksheet molarity, & dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Find the molarity (concentration) of the following solutions: Concentrations and volumes can. Molarity Dilution Worksheet.
From www.pinterest.com
Solutions and Molarity Quiz (Solutes, Solvents, Saturation, and Molarity Dilution Worksheet Calculate the molarity of a solution containing 10.0 grams of sulfuric. Work each of the following problems in the space provided. Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. 1) if i have 340 ml of. Molarity Dilution Worksheet.
From worksheetlistix.z13.web.core.windows.net
Molarity Worksheet 1 Answer Key Chemistry Molarity Dilution Worksheet 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 2) if i add water to 100 ml of a 0.15. Calculate the molarity of a solution containing 10.0 grams of sulfuric. Chemistry ii worksheet molarity, & dilution. Concentrations and volumes can be in any units, as long as both concentrations are. Molarity Dilution Worksheet.
From materialmediaunchain.z5.web.core.windows.net
Molarity And Dilution Worksheet Molarity Dilution Worksheet Chemistry ii worksheet molarity, & dilution. Volume must be in liters! 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Work each of the following. Molarity Dilution Worksheet.
From worksheets.clipart-library.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Molarity Dilution Worksheet Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. Work each of the following problems in the space provided. 2) if i add water to 100 ml of a 0.15. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Volume. Molarity Dilution Worksheet.
From worksheets.clipart-library.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Molarity Dilution Worksheet Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Chemistry ii worksheet molarity, & dilution. Calculate the molarity of a solution containing 10.0 grams of sulfuric. Add more solvent to a. Molarity Dilution Worksheet.
From studylib.net
Molarity Practice Worksheet Molarity Dilution Worksheet Calculate the molarity of a solution containing 10.0 grams of sulfuric. Find the molarity (concentration) of the following solutions: Volume must be in liters! Chemistry ii worksheet molarity, & dilution. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. 1) if i add 25 ml of water to 125. Molarity Dilution Worksheet.
From kidsworksheetfun.com
Molarity And Dilution Worksheet kidsworksheetfun Molarity Dilution Worksheet 2) if i add water to 100 ml of a 0.15. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Find the molarity (concentration) of. Molarity Dilution Worksheet.
From davida.davivienda.com
Molarity Practice Problems Worksheet Printable Word Searches Molarity Dilution Worksheet Work each of the following problems in the space provided. Volume must be in liters! 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add. Molarity Dilution Worksheet.
From worksheets.clipart-library.com
Molarity and Dilution Worksheets Download Free PDF Molar Molarity Dilution Worksheet Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. Work each of the following problems in the space provided. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add more solvent to a solution, thus volume of solution increases while. Molarity Dilution Worksheet.
From studylib.net
Chem 1020 Molarity worksheet, version 2 1. What is the molarity of a Molarity Dilution Worksheet Chemistry ii worksheet molarity, & dilution. Find the molarity (concentration) of the following solutions: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 2) if. Molarity Dilution Worksheet.
From ataglance.randstad.com
Molarity And Dilution Worksheet Printable Calendars AT A GLANCE Molarity Dilution Worksheet Volume must be in liters! Chemistry ii worksheet molarity, & dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Find the molarity (concentration) of the following solutions: Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. Concentrations and volumes. Molarity Dilution Worksheet.
From www.worksheeto.com
7 Best Images of Molarity Worksheet With Answers Molality and Molarity Dilution Worksheet Volume must be in liters! 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Work each of the following problems in the space provided. 2). Molarity Dilution Worksheet.
From learningdbhimmel.z19.web.core.windows.net
Molarity Problems Worksheet With Answers Molarity Dilution Worksheet 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Calculate the molarity of a solution containing 10.0 grams of sulfuric. 1) if i add 25 ml of water to 125 ml. Molarity Dilution Worksheet.
From www.studocu.com
Dilution Worksheet eskandari summer Dilutions Worksheet W 329 Molarity Dilution Worksheet Work each of the following problems in the space provided. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Describe how you would. Molarity Dilution Worksheet.
From worksheets.clipart-library.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Molarity Dilution Worksheet Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Chemistry ii worksheet molarity, & dilution. 2) if i add water to 100 ml of a 0.15. Volume must be in liters! 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what. Molarity Dilution Worksheet.
From worksheets.clipart-library.com
Molarity and Dilutions Practice Worksheet Key Exercises Molarity Dilution Worksheet 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 2) if i add water to 100 ml of a 0.15. Work each of the following problems in the space provided. Chemistry ii worksheet molarity, & dilution. Add more solvent to a solution, thus volume of solution increases while moles of solute. Molarity Dilution Worksheet.
From printablelibswathed.z19.web.core.windows.net
Molarity And Dilution Worksheet Molarity Dilution Worksheet Chemistry ii worksheet molarity, & dilution. Work each of the following problems in the space provided. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. Describe how you would prepare 1.50l. Molarity Dilution Worksheet.
From ataglance.randstad.com
Molarity And Dilution Worksheet Printable Calendars AT A GLANCE Molarity Dilution Worksheet Calculate the molarity of a solution containing 10.0 grams of sulfuric. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. 1) if i add 25 ml of water to 125 ml. Molarity Dilution Worksheet.
From www.studocu.com
Molarity and dilution worksheets 1 Molarity Problems Worksheet M = n Molarity Dilution Worksheet 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Calculate the molarity of a solution containing 10.0 grams of sulfuric. Work each of the following problems in the space provided. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity. Molarity Dilution Worksheet.
From www.e-streetlight.com
Molarity Worksheet Answer Key E Street Light Molarity Dilution Worksheet 2) if i add water to 100 ml of a 0.15. Work each of the following problems in the space provided. Find the molarity (concentration) of the following solutions: Calculate the molarity of a solution containing 10.0 grams of sulfuric. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. Molarity Dilution Worksheet.
From worksheets.clipart-library.com
Free molarity m worksheet, Download Free molarity m worksheet png Molarity Dilution Worksheet Work each of the following problems in the space provided. Find the molarity (concentration) of the following solutions: Chemistry ii worksheet molarity, & dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i have 340 ml of a 0.5 m. Molarity Dilution Worksheet.
From learninglibrarybayer.z13.web.core.windows.net
Molarity And Dilution Worksheet Molarity Dilution Worksheet Volume must be in liters! Chemistry ii worksheet molarity, & dilution. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Find the molarity. Molarity Dilution Worksheet.
From materialzonetomlin.z21.web.core.windows.net
Dilution Problems Worksheets Molarity Dilution Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Find the molarity (concentration) of the following solutions: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Molarity Dilution Worksheet.
From materialoster.z21.web.core.windows.net
Molarity And Dilution Worksheet Molarity Dilution Worksheet Calculate the molarity of a solution containing 10.0 grams of sulfuric. Chemistry ii worksheet molarity, & dilution. Work each of the following problems in the space provided. Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. 2). Molarity Dilution Worksheet.
From dokumen.tips
(PDF) CHEM 12 Practice Worksheet Molarity, Dilution & Dissociation Molarity Dilution Worksheet Concentrations and volumes can be in any units, as long as both concentrations are in the same units as each. 2) if i add water to 100 ml of a 0.15. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Work each of the following problems in the space. Molarity Dilution Worksheet.
From www.pinterest.com
Molarity and Dilutions Notes and Worksheet Set Teaching chemistry Molarity Dilution Worksheet 2) if i add water to 100 ml of a 0.15. Calculate the molarity of a solution containing 10.0 grams of sulfuric. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Add more solvent to a solution, thus volume of solution increases while. Molarity Dilution Worksheet.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Molarity Dilution Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Work each of the following problems in the space provided. Find the molarity (concentration) of the following solutions: 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration. Molarity Dilution Worksheet.
From byveera.blogspot.com
Molarity practice worksheet 13 Science, Chemistry, Solutions Chemistry Molarity Dilution Worksheet Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. 1) if. Molarity Dilution Worksheet.
From www.worksheeto.com
7 Molarity Worksheet With Answers / Molarity Dilution Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Volume must be in liters! Work each of the following problems in the space provided. Add. Molarity Dilution Worksheet.
From www.studypool.com
SOLUTION Dilution Problems Molarity Formula and Dilution Factor PAper Molarity Dilution Worksheet Chemistry ii worksheet molarity, & dilution. Work each of the following problems in the space provided. 2) if i add water to 100 ml of a 0.15. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Find the molarity (concentration) of the following. Molarity Dilution Worksheet.
From learninggarotte.z14.web.core.windows.net
Molarity Dilution Worksheets Molarity Dilution Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Work each of the following problems in the space provided. Calculate the molarity of. Molarity Dilution Worksheet.
From worksheetlive.com
Molarity And Dilution Worksheet Answer Key Worksheet Live Molarity Dilution Worksheet Describe how you would prepare 1.50l of a.25m solution of sodium sulfate. Find the molarity (concentration) of the following solutions: 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Chemistry ii. Molarity Dilution Worksheet.