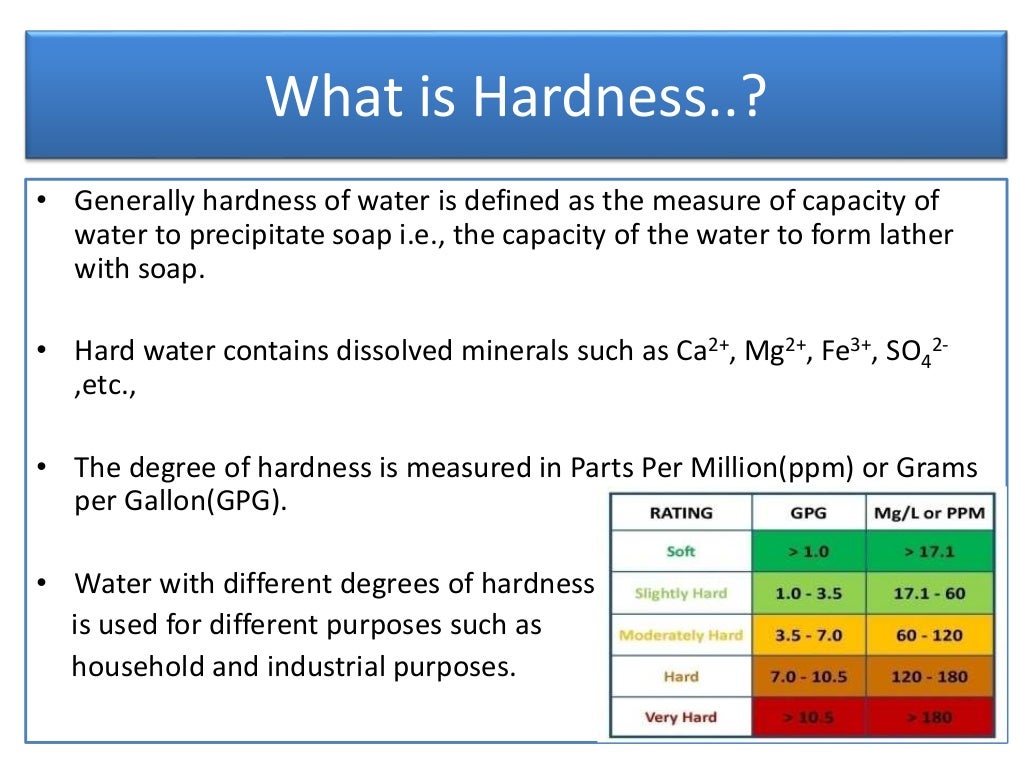
Titration To Find Hardness Of Water . Complexometric titration is one of the best ways of measuring total water hardness. Make a standard solution of edta. Ess was understood to be a measure of the capacity of water to precipit. Therefore the total hardness of water can be determination by edta titration method. Soap is precipitated chiefly by the calcium and. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). At ph around 10 edta easily reacts with both. In this method buffer solution is used for attain suitable condition i.e ph level above 9. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta:
from www.slideshare.net
In this method buffer solution is used for attain suitable condition i.e ph level above 9. Therefore the total hardness of water can be determination by edta titration method. Make a standard solution of edta. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? Ess was understood to be a measure of the capacity of water to precipit. At ph around 10 edta easily reacts with both. Soap is precipitated chiefly by the calcium and. Complexometric titration is one of the best ways of measuring total water hardness. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta:
Determination of hardness of water
Titration To Find Hardness Of Water In this method buffer solution is used for attain suitable condition i.e ph level above 9. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Ess was understood to be a measure of the capacity of water to precipit. Complexometric titration is one of the best ways of measuring total water hardness. In this method buffer solution is used for attain suitable condition i.e ph level above 9. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). Therefore the total hardness of water can be determination by edta titration method. At ph around 10 edta easily reacts with both. Make a standard solution of edta. Soap is precipitated chiefly by the calcium and. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration?
From www.youtube.com
Hardness Buret Titration YouTube Titration To Find Hardness Of Water Ess was understood to be a measure of the capacity of water to precipit. Complexometric titration is one of the best ways of measuring total water hardness. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. Soap is precipitated chiefly by the calcium and. What would be the. Titration To Find Hardness Of Water.
From www.youtube.com
How to determine hardness of water by EDTA method? (Procedure and Titration To Find Hardness Of Water Soap is precipitated chiefly by the calcium and. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). At ph around 10 edta easily reacts with both. Therefore the total hardness of water. Titration To Find Hardness Of Water.
From www.slideserve.com
PPT EDTA Titration of Water PowerPoint Presentation, free download Titration To Find Hardness Of Water Ess was understood to be a measure of the capacity of water to precipit. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: In this. Titration To Find Hardness Of Water.
From www.youtube.com
Determination of Hardness of Water by EDTA method Standardization of Titration To Find Hardness Of Water There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Soap is precipitated chiefly by the calcium and. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). In this method buffer solution is used for attain suitable condition i.e ph level. Titration To Find Hardness Of Water.
From www.youtube.com
Unit 1 Estimation of Hardness of water by EDTA method YouTube Titration To Find Hardness Of Water Ess was understood to be a measure of the capacity of water to precipit. In this method buffer solution is used for attain suitable condition i.e ph level above 9. Therefore the total hardness of water can be determination by edta titration method. Complexometric titration is one of the best ways of measuring total water hardness. There are 3 steps. Titration To Find Hardness Of Water.
From www.youtube.com
Determination of Hardness of water by EDTA Method YouTube Titration To Find Hardness Of Water Complexometric titration is one of the best ways of measuring total water hardness. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). In this method buffer solution is used for attain suitable condition i.e ph level above 9. At ph around 10 edta easily reacts with both. Therefore the total hardness of water can. Titration To Find Hardness Of Water.
From www.slideserve.com
PPT Hardness of Water PowerPoint Presentation, free download ID2279522 Titration To Find Hardness Of Water Therefore the total hardness of water can be determination by edta titration method. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. Soap is precipitated chiefly by the calcium and. What would be the general features of an indicator that could be used to determine the endpoint of. Titration To Find Hardness Of Water.
From www.youtube.com
Determination of Hardness of Water_A Complete Procedure (ASTM D112617 Titration To Find Hardness Of Water There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Ess was understood to be a measure of the capacity of water to precipit. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). At ph around 10 edta easily reacts with. Titration To Find Hardness Of Water.
From www.youtube.com
EDTA TITRATION DETERMINATION OF TOTAL HARDNESS OF WATER YouTube Titration To Find Hardness Of Water There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Soap is precipitated chiefly by the calcium and. At ph around 10 edta easily reacts with both. In this method buffer solution is used for attain suitable condition i.e ph level above 9. Make a standard solution. Titration To Find Hardness Of Water.
From www.scribd.com
Water Hardness Lab Report Titration Chemistry Titration To Find Hardness Of Water Complexometric titration is one of the best ways of measuring total water hardness. Make a standard solution of edta. Therefore the total hardness of water can be determination by edta titration method. Ess was understood to be a measure of the capacity of water to precipit. There are 3 steps to determining the concentration of calcium and magnesium ions in. Titration To Find Hardness Of Water.
From mavink.com
Titration Water Hardness Test Kit Titration To Find Hardness Of Water Complexometric titration is one of the best ways of measuring total water hardness. Ess was understood to be a measure of the capacity of water to precipit. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: In this method buffer solution is used for attain suitable. Titration To Find Hardness Of Water.
From www.youtube.com
Water Hardness (EDTA) Titration Calculations Example YouTube Titration To Find Hardness Of Water At ph around 10 edta easily reacts with both. In this method buffer solution is used for attain suitable condition i.e ph level above 9. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration. Titration To Find Hardness Of Water.
From www.youtube.com
Determination of Hardness in Water Sample Complexometric titration Titration To Find Hardness Of Water Therefore the total hardness of water can be determination by edta titration method. Complexometric titration is one of the best ways of measuring total water hardness. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? There are 3 steps to determining the concentration of calcium and magnesium. Titration To Find Hardness Of Water.
From studylib.net
Experiment 6 EDTA Titration of Hardness of Water Titration To Find Hardness Of Water Therefore the total hardness of water can be determination by edta titration method. At ph around 10 edta easily reacts with both. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? Make a standard solution of edta. In this method buffer solution is used for attain suitable. Titration To Find Hardness Of Water.
From www.youtube.com
Determining Water Hardness by Complexometric Titration YouTube Titration To Find Hardness Of Water What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. Complexometric titration is one of the best ways of measuring total water hardness. Ess was understood to. Titration To Find Hardness Of Water.
From www.slideserve.com
PPT EDTA Titration of Water PowerPoint Presentation, free download Titration To Find Hardness Of Water Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). In this method buffer solution is used for attain suitable condition i.e ph level above 9. Make a standard solution of edta. Soap is precipitated chiefly by the calcium and. This sop describes the procedure for measuring hardness by titration with standard edta solution to. Titration To Find Hardness Of Water.
From www.chegg.com
Determination of Water Hardness by Titration Lab Titration To Find Hardness Of Water What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? Ess was understood to be a measure of the capacity of water to precipit. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). Therefore the total hardness of water can be determination. Titration To Find Hardness Of Water.
From mavink.com
Edta Titration For Water Hardness Titration To Find Hardness Of Water This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. Soap is precipitated chiefly by the calcium and. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Therefore the total hardness of water can be. Titration To Find Hardness Of Water.
From www.slideshare.net
Determination of hardness of water Titration To Find Hardness Of Water Make a standard solution of edta. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). At ph around 10 edta easily reacts with both. In this method buffer solution is used for attain suitable condition i.e ph level above 9. Therefore the total hardness of water can be determination by edta titration method. What. Titration To Find Hardness Of Water.
From www.slideshare.net
Lab5 determination of hardness of water Titration To Find Hardness Of Water Therefore the total hardness of water can be determination by edta titration method. At ph around 10 edta easily reacts with both. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. Complexometric titration is one of the best ways of measuring total water hardness. There are 3 steps. Titration To Find Hardness Of Water.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Titration To Find Hardness Of Water Ess was understood to be a measure of the capacity of water to precipit. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Make a standard solution of edta. Soap is precipitated chiefly by the calcium and. At ph around 10 edta easily reacts with both.. Titration To Find Hardness Of Water.
From www.youtube.com
EDTA Titration method to find the hardness of water by Sityog Institute Titration To Find Hardness Of Water Ess was understood to be a measure of the capacity of water to precipit. Make a standard solution of edta. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: What would be the general features of an indicator that could be used to determine the endpoint. Titration To Find Hardness Of Water.
From www.youtube.com
WATER HARDNESS DETERMINATION VIA TITRATION REACTIONS YouTube Titration To Find Hardness Of Water Complexometric titration is one of the best ways of measuring total water hardness. Make a standard solution of edta. Soap is precipitated chiefly by the calcium and. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. Therefore the total hardness of water can be determination by edta titration. Titration To Find Hardness Of Water.
From www.youtube.com
Determination of water hardness (titration) YouTube Titration To Find Hardness Of Water Therefore the total hardness of water can be determination by edta titration method. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). In this method buffer solution is used for attain suitable condition i.e ph level above 9. Soap is precipitated chiefly by the calcium and. This sop describes the procedure for measuring hardness. Titration To Find Hardness Of Water.
From www.slideserve.com
PPT Water Hardness Determination with EDTA PowerPoint Presentation Titration To Find Hardness Of Water Make a standard solution of edta. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? Soap is precipitated chiefly by the calcium and. In this method buffer solution is used for attain suitable condition i.e ph level above 9. Therefore the total hardness of water can be. Titration To Find Hardness Of Water.
From www.researchgate.net
(PDF) Determination of the hardness of tap water using EDTA titration. Titration To Find Hardness Of Water What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? Soap is precipitated chiefly by the calcium and. Make a standard solution of edta. Therefore the total hardness of water can be determination by edta titration method. Water hardness can be readily determined by titration with the chelating. Titration To Find Hardness Of Water.
From studylib.net
Experiment 6 EDTA Titration of the Hardness of Water Titration To Find Hardness Of Water Complexometric titration is one of the best ways of measuring total water hardness. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? Water hardness can be. Titration To Find Hardness Of Water.
From www.youtube.com
EDTA Titration Complexomatric Titration Hardness of Water Titration To Find Hardness Of Water Soap is precipitated chiefly by the calcium and. Complexometric titration is one of the best ways of measuring total water hardness. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: This. Titration To Find Hardness Of Water.
From www.youtube.com
Determination of Hardness by Complexometric/ EDTA Method_Water and its Titration To Find Hardness Of Water Complexometric titration is one of the best ways of measuring total water hardness. At ph around 10 edta easily reacts with both. In this method buffer solution is used for attain suitable condition i.e ph level above 9. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration?. Titration To Find Hardness Of Water.
From www.youtube.com
Determination of total hardness of water by complexometric titration Titration To Find Hardness Of Water There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). Ess was understood to be a measure of the capacity of water to precipit. Make a standard solution of edta. At ph. Titration To Find Hardness Of Water.
From www.youtube.com
Titrate water with EDTA using EBT to find Hardness of Water 🤔? Easy Titration To Find Hardness Of Water There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: In this method buffer solution is used for attain suitable condition i.e ph level above 9. Complexometric titration is one of the best ways of measuring total water hardness. Water hardness can be readily determined by titration. Titration To Find Hardness Of Water.
From www.scribd.com
Determination of Hardness of Water PDF Chemistry Titration Titration To Find Hardness Of Water At ph around 10 edta easily reacts with both. Complexometric titration is one of the best ways of measuring total water hardness. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Ess was understood to be a measure of the capacity of water to precipit. Water. Titration To Find Hardness Of Water.
From www.youtube.com
Estimation of hardness of water II EDTA complexometric titration II Titration To Find Hardness Of Water Soap is precipitated chiefly by the calcium and. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Therefore the total hardness of water can be determination by edta titration method. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint. Titration To Find Hardness Of Water.
From wholeworldwater.co
Hard Water Whole World Water Titration To Find Hardness Of Water Soap is precipitated chiefly by the calcium and. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). Complexometric titration is one of the best ways of measuring total water hardness. This sop describes the procedure for measuring hardness by titration with standard edta solution to endpoint indicated by a color change. There are 3. Titration To Find Hardness Of Water.
From www.youtube.com
EDTA Titrimetric Method (Experiment) Measurement of Total hardness, Ca Titration To Find Hardness Of Water Make a standard solution of edta. At ph around 10 edta easily reacts with both. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? There are 3 steps to determining the concentration. Titration To Find Hardness Of Water.