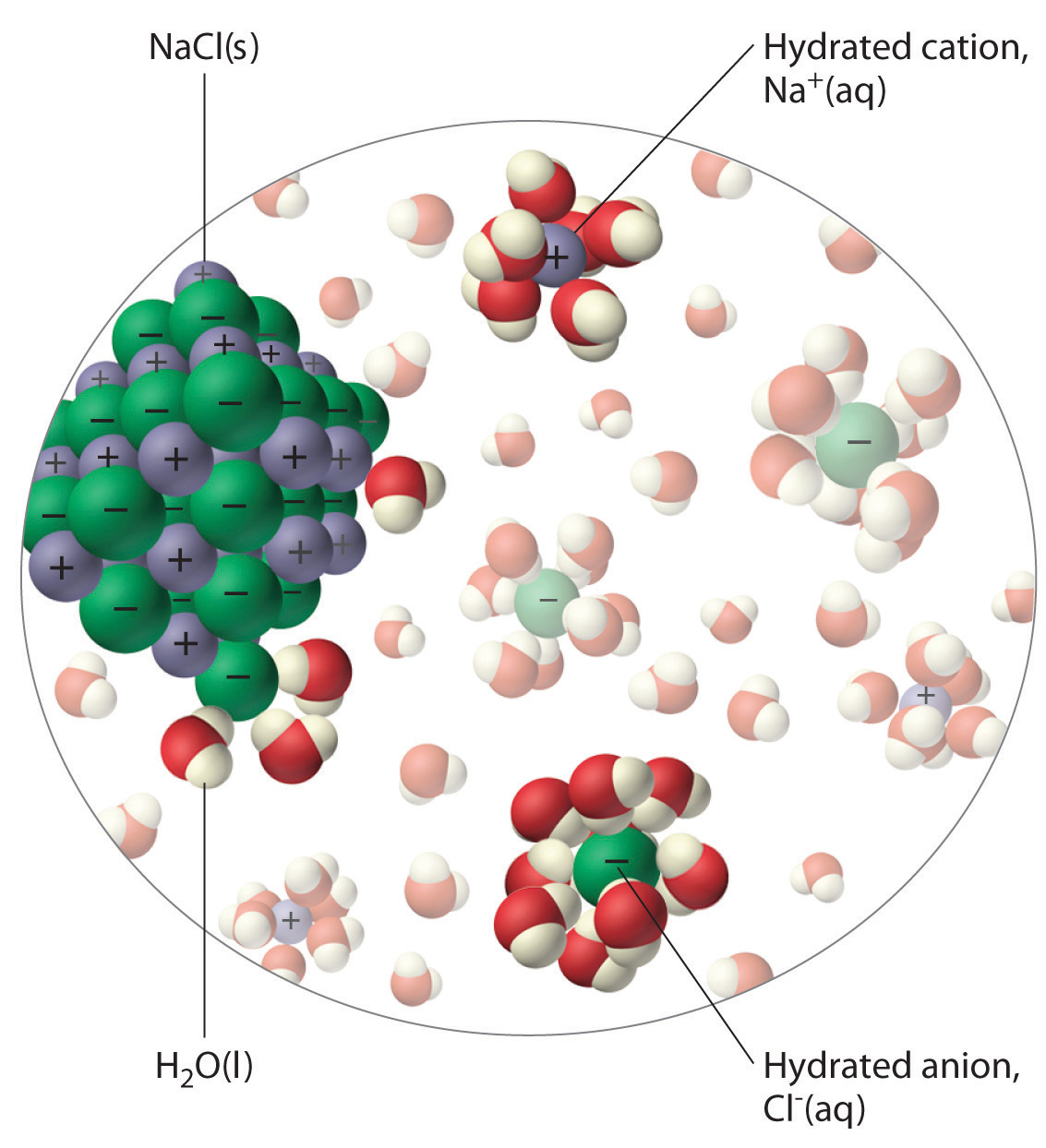
Does The Oil Dissolve In Water . The electronegativity difference between these two elements is about 1.24, meaning it is very polar. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. oil floats on water because it is less dense or has lower specific gravity. That causes oil and water to form two separate layers. view full lesson: Unlike many other substances such as fruit juice, food dyes. Water is polar because of the bonds between oxygen and hydrogen are polar. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. the water molecules attract each other, and the oil molecules stick together.
from communitystudentlearning.org
Water is polar because of the bonds between oxygen and hydrogen are polar. oil floats on water because it is less dense or has lower specific gravity. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. the water molecules attract each other, and the oil molecules stick together. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. view full lesson: Unlike many other substances such as fruit juice, food dyes. That causes oil and water to form two separate layers.
What Does Oil Dissolve In What Dissolves Oil?
Does The Oil Dissolve In Water That causes oil and water to form two separate layers. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. view full lesson: oil floats on water because it is less dense or has lower specific gravity. Water is polar because of the bonds between oxygen and hydrogen are polar. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. That causes oil and water to form two separate layers. Unlike many other substances such as fruit juice, food dyes. the water molecules attract each other, and the oil molecules stick together.
From www.youtube.com
Science Experiment Does oil dissolve in water? YouTube Does The Oil Dissolve In Water the water molecules attract each other, and the oil molecules stick together. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. That causes oil and water to form two separate layers. view full lesson: Water is polar because of the bonds between oxygen and hydrogen are polar. Unlike many other substances such. Does The Oil Dissolve In Water.
From www.youtube.com
Boiling point of oil is greater than water ? YouTube Does The Oil Dissolve In Water the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. oil floats on water because it is less dense or has lower specific gravity. the water molecules attract each other, and the oil molecules stick together. Water molecules pack closer together, so they sink to. Does The Oil Dissolve In Water.
From www.youtube.com
Why Oil and Water Don’t Mix Oil and Water Experiment Explained How Does The Oil Dissolve In Water Unlike many other substances such as fruit juice, food dyes. That causes oil and water to form two separate layers. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil. Does The Oil Dissolve In Water.
From cobyfersrobinson.blogspot.com
Density of Oil and Water Does The Oil Dissolve In Water Water is polar because of the bonds between oxygen and hydrogen are polar. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. That causes oil and water to form two separate layers. Unlike many other substances such as fruit juice, food dyes. oil floats on. Does The Oil Dissolve In Water.
From www.youtube.com
Solubility of salt and vegetable oil in water YouTube Does The Oil Dissolve In Water That causes oil and water to form two separate layers. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. the water molecules attract each other, and the oil molecules stick together. The electronegativity difference between these two elements is about 1.24, meaning it is very. Does The Oil Dissolve In Water.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Does The Oil Dissolve In Water That causes oil and water to form two separate layers. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. the water molecules attract each other, and the oil molecules stick together. view full lesson: Unlike many other substances such as fruit juice, food dyes. The electronegativity difference between. Does The Oil Dissolve In Water.
From readingandwritingprojectcom.web.fc2.com
oil is soluble in water Does The Oil Dissolve In Water view full lesson: the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. oil floats on water because it is less dense or has lower specific gravity. That causes oil and water to form two separate layers. Unlike many other substances such as fruit juice,. Does The Oil Dissolve In Water.
From sapsnj.com
What Does Oil Dissolve In Solubility Does The Oil Dissolve In Water view full lesson: That causes oil and water to form two separate layers. Water is polar because of the bonds between oxygen and hydrogen are polar. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. the water molecules attract each other, and the oil. Does The Oil Dissolve In Water.
From phys.org
Study reveals sunlight can help dissolve oil into seawater Does The Oil Dissolve In Water Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. the water molecules attract each other, and the oil molecules stick together. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. view full lesson: That causes oil and water to form two separate. Does The Oil Dissolve In Water.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? in Does The Oil Dissolve In Water the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. oil floats on water because it is less dense or has lower specific gravity. Water is polar because of the bonds between oxygen and hydrogen are polar. That causes oil and water to form two separate. Does The Oil Dissolve In Water.
From exohpqwmj.blob.core.windows.net
Which Crystals Dissolve In Water at Vivian Lamotte blog Does The Oil Dissolve In Water the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. the water molecules attract each other, and the oil molecules stick together. oil floats on water because it is less dense or has lower specific gravity. Water molecules pack closer together, so they sink to. Does The Oil Dissolve In Water.
From communitystudentlearning.org
What Does Oil Dissolve In What Dissolves Oil? Does The Oil Dissolve In Water That causes oil and water to form two separate layers. oil floats on water because it is less dense or has lower specific gravity. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. the basic answer is that water molecules attract each other and so clump together forcing almost all of the. Does The Oil Dissolve In Water.
From www.chegg.com
Solved Why Doesn't Oil Dissolve In Water? ? Oil Molecules... Does The Oil Dissolve In Water That causes oil and water to form two separate layers. view full lesson: Water is polar because of the bonds between oxygen and hydrogen are polar. oil floats on water because it is less dense or has lower specific gravity. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. Water molecules pack. Does The Oil Dissolve In Water.
From llmontessori.org
What Does Oil Dissolve In — Support Science Journalism Does The Oil Dissolve In Water view full lesson: Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. Unlike many other substances such as fruit juice, food dyes. That causes oil and water to form two separate layers. oil floats on water because it is less dense or has lower specific gravity. The electronegativity. Does The Oil Dissolve In Water.
From yesdirt.com
Does Oil Dissolve In Water? (ANSWERED) Yes Dirt Does The Oil Dissolve In Water The electronegativity difference between these two elements is about 1.24, meaning it is very polar. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. Unlike many other substances such as fruit juice, food dyes. That causes oil and water to form two separate layers. Water molecules. Does The Oil Dissolve In Water.
From www.slideserve.com
PPT CHEMICAL BONDING PowerPoint Presentation ID1990199 Does The Oil Dissolve In Water the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. That causes oil and water to form two separate layers. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. oil floats on water because it is. Does The Oil Dissolve In Water.
From dfmedica.com.br
What Does Oil Dissolve In — Aromatic Formulation Does The Oil Dissolve In Water The electronegativity difference between these two elements is about 1.24, meaning it is very polar. That causes oil and water to form two separate layers. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. Water molecules pack closer together, so they sink to the bottom, leaving. Does The Oil Dissolve In Water.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download Does The Oil Dissolve In Water Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. Unlike many other substances such as fruit juice, food dyes. Water is polar because of the bonds between oxygen and hydrogen are polar. view full lesson: the water molecules attract each other, and the oil molecules stick together. . Does The Oil Dissolve In Water.
From www.youtube.com
Why Does This Powder Only Dissolve In Cold Water? YouTube Does The Oil Dissolve In Water Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. view full lesson: Water is polar because of the bonds between oxygen and hydrogen are polar. That causes oil and water to form two separate layers. oil floats on water because it is less dense or has lower specific. Does The Oil Dissolve In Water.
From lifeholistically.com
how to properly disperse essential oils in water Archives Does The Oil Dissolve In Water the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. Water is polar because of the bonds between oxygen and hydrogen are polar. That causes oil and water to form two separate layers. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting. Does The Oil Dissolve In Water.
From brainly.in
the substance that will not dissolve in water Brainly.in Does The Oil Dissolve In Water Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. That causes oil and water to form two separate layers. Unlike many other substances such as fruit juice, food dyes. the water molecules attract each other, and the oil molecules stick together. oil floats on water because it is. Does The Oil Dissolve In Water.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Does The Oil Dissolve In Water the water molecules attract each other, and the oil molecules stick together. oil floats on water because it is less dense or has lower specific gravity. view full lesson: That causes oil and water to form two separate layers. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the. Does The Oil Dissolve In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Does The Oil Dissolve In Water view full lesson: the water molecules attract each other, and the oil molecules stick together. oil floats on water because it is less dense or has lower specific gravity. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. the basic answer is that water molecules attract each other and so. Does The Oil Dissolve In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Does The Oil Dissolve In Water Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. That causes oil and water to form two separate layers. oil floats on water because it is less dense or has lower specific gravity. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. . Does The Oil Dissolve In Water.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize Does The Oil Dissolve In Water view full lesson: Unlike many other substances such as fruit juice, food dyes. Water is polar because of the bonds between oxygen and hydrogen are polar. oil floats on water because it is less dense or has lower specific gravity. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the. Does The Oil Dissolve In Water.
From www.youtube.com
oil does not dissolve in water YouTube Does The Oil Dissolve In Water Unlike many other substances such as fruit juice, food dyes. That causes oil and water to form two separate layers. the water molecules attract each other, and the oil molecules stick together. oil floats on water because it is less dense or has lower specific gravity. The electronegativity difference between these two elements is about 1.24, meaning it. Does The Oil Dissolve In Water.
From slideplayer.com
Water’s Unique Property Lab ppt download Does The Oil Dissolve In Water Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. the water molecules attract each other, and the oil molecules stick together. Water is polar because of the bonds between oxygen and hydrogen are polar. That causes oil and water to form two separate layers. view full lesson: . Does The Oil Dissolve In Water.
From sciencing.com
Molecular Activity of Water Vs. Oil Sciencing Does The Oil Dissolve In Water Water is polar because of the bonds between oxygen and hydrogen are polar. the water molecules attract each other, and the oil molecules stick together. That causes oil and water to form two separate layers. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. The. Does The Oil Dissolve In Water.
From www.youtube.com
Why oil does not dissolve in water Polarity of water YouTube Does The Oil Dissolve In Water the water molecules attract each other, and the oil molecules stick together. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. view full lesson: That causes oil and water to form two separate layers. The electronegativity difference between these two elements is about 1.24,. Does The Oil Dissolve In Water.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Does The Oil Dissolve In Water The electronegativity difference between these two elements is about 1.24, meaning it is very polar. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. Water is polar. Does The Oil Dissolve In Water.
From www.chegg.com
Solved I would expect the compound NaBr to (select all that Does The Oil Dissolve In Water Unlike many other substances such as fruit juice, food dyes. Water is polar because of the bonds between oxygen and hydrogen are polar. oil floats on water because it is less dense or has lower specific gravity. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out. Does The Oil Dissolve In Water.
From mechanic8daasuge.z21.web.core.windows.net
Why Is My Oil Watery Does The Oil Dissolve In Water the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. view full lesson: the water molecules attract each other, and the oil molecules stick together. Unlike many other substances such as fruit juice, food dyes. oil floats on water because it is less dense. Does The Oil Dissolve In Water.
From www.youtube.com
Why Salt (NaCl) Does Not Dissolve in Oil Salt and Oil Experiment Is Does The Oil Dissolve In Water oil floats on water because it is less dense or has lower specific gravity. Water molecules pack closer together, so they sink to the bottom, leaving oil sitting on top of the water. That causes oil and water to form two separate layers. Unlike many other substances such as fruit juice, food dyes. Water is polar because of the. Does The Oil Dissolve In Water.
From www.youtube.com
Dissolving oil in water YouTube Does The Oil Dissolve In Water the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. oil floats on water because it is less dense or has lower specific gravity. Water is polar because of the bonds. Does The Oil Dissolve In Water.
From studypositivity.z21.web.core.windows.net
How Does Water Dissolve Substances Does The Oil Dissolve In Water oil floats on water because it is less dense or has lower specific gravity. The electronegativity difference between these two elements is about 1.24, meaning it is very polar. the basic answer is that water molecules attract each other and so clump together forcing almost all of the oil out of. That causes oil and water to form. Does The Oil Dissolve In Water.