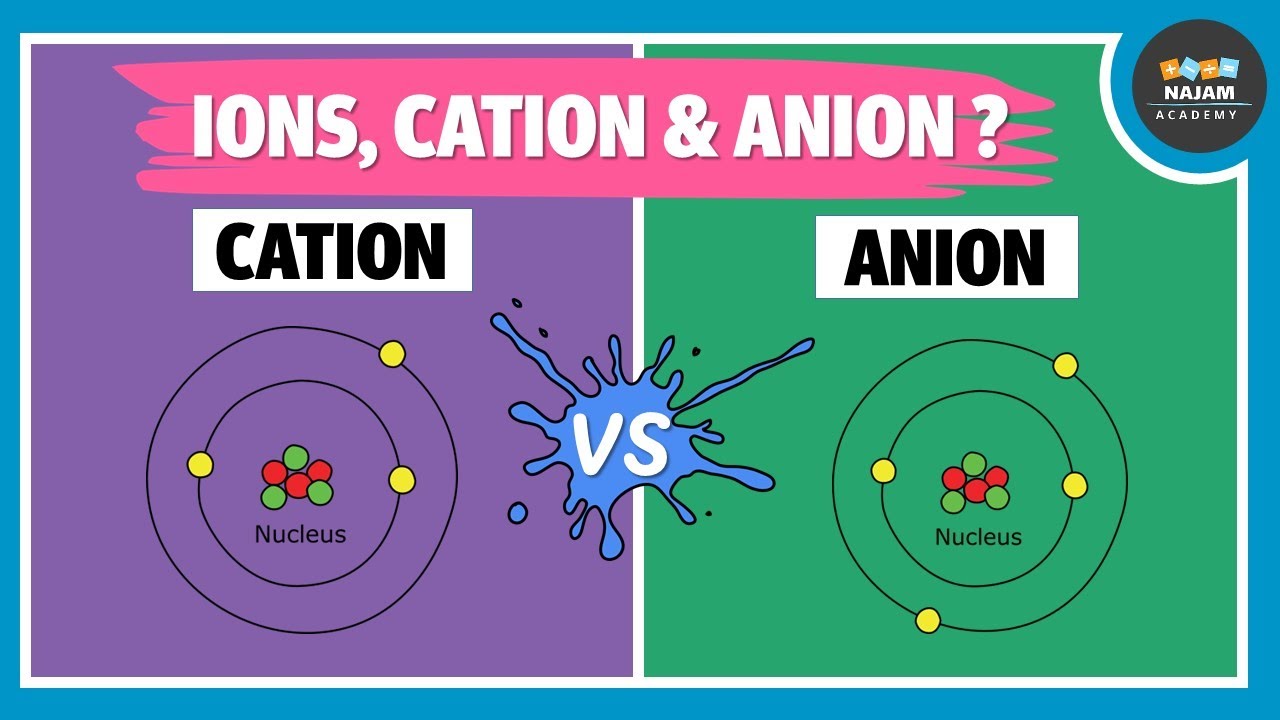
What Does A Cation Look Like . K + (potassium ion)) while an anion is a negatively charged ion. Anions are ions that are negatively charged. a cation is a positively charged ion with fewer electrons than protons [2] (e.g. Explain how neutral atoms ionize to form cations. when writing the formula of a compound, the cation is listed before the anion. Ions are charged atoms or molecules. Cations are named according to the parent. The word cation comes from the greek. cations are formed by the loss of one or two electrons from an element. If a balanced atom gains one or more electrons, it will become a negatively charged anion. Because one or more electrons are removed to. A cation is an ion that has lost one or more electrons, giving a net positive charge. Groups 1 and 2 elements form cations. a cation is an ionic species with a positive charge. For example, in nacl, the sodium atom acts as the cation, while the.
from quizunderfires.z21.web.core.windows.net
If a balanced atom gains one or more electrons, it will become a negatively charged anion. Because one or more electrons are removed to. Ions are charged atoms or molecules. A cation is an ion that has lost one or more electrons, giving a net positive charge. Anions are ions that are negatively charged. a cation is an ionic species with a positive charge. cations are formed by the loss of one or two electrons from an element. Determine the charges achieved when main group. when writing the formula of a compound, the cation is listed before the anion. For example, in nacl, the sodium atom acts as the cation, while the.
How Is A Anion Formed
What Does A Cation Look Like Cations are named according to the parent. cations are formed by the loss of one or two electrons from an element. Explain how neutral atoms ionize to form cations. K + (potassium ion)) while an anion is a negatively charged ion. a cation is a positively charged ion with fewer electrons than protons [2] (e.g. cations are ions that are positively charged. a cation is an ionic species with a positive charge. A cation is an ion that has lost one or more electrons, giving a net positive charge. The word cation comes from the greek. If a balanced atom loses one or more electrons, it will become a positively charged cation. If a balanced atom gains one or more electrons, it will become a negatively charged anion. Cations are named according to the parent. when writing the formula of a compound, the cation is listed before the anion. Groups 1 and 2 elements form cations. For example, in nacl, the sodium atom acts as the cation, while the. Anions are ions that are negatively charged.
From www.slideserve.com
PPT Elements PowerPoint Presentation, free download ID1588776 What Does A Cation Look Like a cation is an ionic species with a positive charge. a cation is a positively charged ion with fewer electrons than protons [2] (e.g. Cations are named according to the parent. Anions are ions that are negatively charged. Groups 1 and 2 elements form cations. The word cation comes from the greek. For example, in nacl, the sodium. What Does A Cation Look Like.
From eliasmeowescobar.blogspot.com
Is Manganese a Cation or Anion What Does A Cation Look Like A cation is an ion that has lost one or more electrons, giving a net positive charge. a cation is an ionic species with a positive charge. Ions are charged atoms or molecules. cations are ions that are positively charged. If a balanced atom gains one or more electrons, it will become a negatively charged anion. cations. What Does A Cation Look Like.
From www.reddit.com
If it doesn't look like a chair to you, that's because it had some What Does A Cation Look Like Because one or more electrons are removed to. Anions are ions that are negatively charged. cations are formed by the loss of one or two electrons from an element. If a balanced atom gains one or more electrons, it will become a negatively charged anion. Explain how neutral atoms ionize to form cations. Cations are named according to the. What Does A Cation Look Like.
From www.difference101.com
Cation vs. Anion 7 Key Differences, Pros & Cons, Examples Difference 101 What Does A Cation Look Like For example, in nacl, the sodium atom acts as the cation, while the. Anions are ions that are negatively charged. Determine the charges achieved when main group. a cation is an ionic species with a positive charge. Because one or more electrons are removed to. Cations are named according to the parent. Explain how neutral atoms ionize to form. What Does A Cation Look Like.
From thefinchandpea.com
cation What Does A Cation Look Like Because one or more electrons are removed to. Anions are ions that are negatively charged. cations are formed by the loss of one or two electrons from an element. A cation is an ion that has lost one or more electrons, giving a net positive charge. Determine the charges achieved when main group. If a balanced atom gains one. What Does A Cation Look Like.
From learningschoolscrotums.z14.web.core.windows.net
Times Chart Up To 10 What Does A Cation Look Like a cation is a positively charged ion with fewer electrons than protons [2] (e.g. Because one or more electrons are removed to. Groups 1 and 2 elements form cations. cations are ions that are positively charged. The word cation comes from the greek. Cations are named according to the parent. Anions are ions that are negatively charged. K. What Does A Cation Look Like.
From pediaa.com
Difference Between Cation and Anion What Does A Cation Look Like Ions are charged atoms or molecules. cations are formed by the loss of one or two electrons from an element. K + (potassium ion)) while an anion is a negatively charged ion. For example, in nacl, the sodium atom acts as the cation, while the. cations are ions that are positively charged. a cation is a positively. What Does A Cation Look Like.
From learningmediaallan99.z13.web.core.windows.net
How To Ions Form What Does A Cation Look Like cations are formed by the loss of one or two electrons from an element. a cation is an ionic species with a positive charge. Explain how neutral atoms ionize to form cations. Ions are charged atoms or molecules. cations are ions that are positively charged. when writing the formula of a compound, the cation is listed. What Does A Cation Look Like.
From www.reddit.com
Looks like you’re in need of an Ed. U. Cation. r/dankmemes What Does A Cation Look Like Explain how neutral atoms ionize to form cations. Groups 1 and 2 elements form cations. Determine the charges achieved when main group. Anions are ions that are negatively charged. Ions are charged atoms or molecules. For example, in nacl, the sodium atom acts as the cation, while the. A cation is an ion that has lost one or more electrons,. What Does A Cation Look Like.
From study.com
Cation Definition & Examples Video & Lesson Transcript What Does A Cation Look Like a cation is an ionic species with a positive charge. Determine the charges achieved when main group. when writing the formula of a compound, the cation is listed before the anion. cations are formed by the loss of one or two electrons from an element. If a balanced atom loses one or more electrons, it will become. What Does A Cation Look Like.
From knoxvillewatertreatment.com
What Does A Cation Look Like The word cation comes from the greek. cations are formed by the loss of one or two electrons from an element. a cation is an ionic species with a positive charge. Explain how neutral atoms ionize to form cations. Determine the charges achieved when main group. Because one or more electrons are removed to. cations are ions. What Does A Cation Look Like.
From ar.inspiredpencil.com
Hydrogen Ion What Does A Cation Look Like If a balanced atom gains one or more electrons, it will become a negatively charged anion. Ions are charged atoms or molecules. cations are ions that are positively charged. If a balanced atom loses one or more electrons, it will become a positively charged cation. when writing the formula of a compound, the cation is listed before the. What Does A Cation Look Like.
From slideplayer.com
Turning chemistry into algebra ppt download What Does A Cation Look Like cations are formed by the loss of one or two electrons from an element. Anions are ions that are negatively charged. cations are ions that are positively charged. If a balanced atom gains one or more electrons, it will become a negatively charged anion. Because one or more electrons are removed to. A cation is an ion that. What Does A Cation Look Like.
From azsage.com
How Does a Lithium Cation Compare to a Lithium Atom [2023] AZSAGE What Does A Cation Look Like Explain how neutral atoms ionize to form cations. K + (potassium ion)) while an anion is a negatively charged ion. Cations are named according to the parent. Determine the charges achieved when main group. Anions are ions that are negatively charged. cations are formed by the loss of one or two electrons from an element. cations are ions. What Does A Cation Look Like.
From www.youtube.com
Molecular Orbital Theory of Allylic Cations and Radicals YouTube What Does A Cation Look Like Because one or more electrons are removed to. cations are formed by the loss of one or two electrons from an element. Determine the charges achieved when main group. Anions are ions that are negatively charged. K + (potassium ion)) while an anion is a negatively charged ion. Explain how neutral atoms ionize to form cations. Ions are charged. What Does A Cation Look Like.
From www.worldatlas.com
What is the Difference Between a Cation and an Anion? What Does A Cation Look Like Determine the charges achieved when main group. Groups 1 and 2 elements form cations. If a balanced atom gains one or more electrons, it will become a negatively charged anion. when writing the formula of a compound, the cation is listed before the anion. If a balanced atom loses one or more electrons, it will become a positively charged. What Does A Cation Look Like.
From lessoncampuspolypide.z5.web.core.windows.net
How To Find Polyatomic Ion Formulas What Does A Cation Look Like a cation is a positively charged ion with fewer electrons than protons [2] (e.g. Ions are charged atoms or molecules. If a balanced atom gains one or more electrons, it will become a negatively charged anion. Determine the charges achieved when main group. cations are formed by the loss of one or two electrons from an element. If. What Does A Cation Look Like.
From slideplayer.com
The Structure of Matter Chapter 6 ppt download What Does A Cation Look Like For example, in nacl, the sodium atom acts as the cation, while the. Explain how neutral atoms ionize to form cations. a cation is an ionic species with a positive charge. If a balanced atom gains one or more electrons, it will become a negatively charged anion. Cations are named according to the parent. a cation is a. What Does A Cation Look Like.
From quizunderfires.z21.web.core.windows.net
How Is A Anion Formed What Does A Cation Look Like a cation is an ionic species with a positive charge. Determine the charges achieved when main group. For example, in nacl, the sodium atom acts as the cation, while the. K + (potassium ion)) while an anion is a negatively charged ion. If a balanced atom gains one or more electrons, it will become a negatively charged anion. Cations. What Does A Cation Look Like.
From slideplayer.com
What are the functions of the skeletal system? 10/8 ppt download What Does A Cation Look Like Anions are ions that are negatively charged. cations are ions that are positively charged. Ions are charged atoms or molecules. Cations are named according to the parent. If a balanced atom gains one or more electrons, it will become a negatively charged anion. A cation is an ion that has lost one or more electrons, giving a net positive. What Does A Cation Look Like.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences What Does A Cation Look Like Cations are named according to the parent. If a balanced atom gains one or more electrons, it will become a negatively charged anion. If a balanced atom loses one or more electrons, it will become a positively charged cation. Groups 1 and 2 elements form cations. A cation is an ion that has lost one or more electrons, giving a. What Does A Cation Look Like.
From www.geeksforgeeks.org
Cations and Anions Difference between Cations and Anions What Does A Cation Look Like a cation is a positively charged ion with fewer electrons than protons [2] (e.g. K + (potassium ion)) while an anion is a negatively charged ion. Anions are ions that are negatively charged. Groups 1 and 2 elements form cations. when writing the formula of a compound, the cation is listed before the anion. cations are ions. What Does A Cation Look Like.
From www.youtube.com
Medical vocabulary What does Cations, Divalent mean YouTube What Does A Cation Look Like a cation is an ionic species with a positive charge. Cations are named according to the parent. If a balanced atom loses one or more electrons, it will become a positively charged cation. The word cation comes from the greek. Explain how neutral atoms ionize to form cations. A cation is an ion that has lost one or more. What Does A Cation Look Like.
From sciencetolf.weebly.com
Periodic table with charged ions sciencetolf What Does A Cation Look Like The word cation comes from the greek. cations are formed by the loss of one or two electrons from an element. Anions are ions that are negatively charged. If a balanced atom gains one or more electrons, it will become a negatively charged anion. A cation is an ion that has lost one or more electrons, giving a net. What Does A Cation Look Like.
From dxonqyrdw.blob.core.windows.net
What Is An Ion Made Of at Kathryn Allard blog What Does A Cation Look Like Groups 1 and 2 elements form cations. Anions are ions that are negatively charged. Determine the charges achieved when main group. A cation is an ion that has lost one or more electrons, giving a net positive charge. For example, in nacl, the sodium atom acts as the cation, while the. cations are ions that are positively charged. Ions. What Does A Cation Look Like.
From www.youtube.com
What is an Ion Definition, Formation ,Examples and types of ions What Does A Cation Look Like K + (potassium ion)) while an anion is a negatively charged ion. cations are formed by the loss of one or two electrons from an element. A cation is an ion that has lost one or more electrons, giving a net positive charge. Groups 1 and 2 elements form cations. Anions are ions that are negatively charged. Ions are. What Does A Cation Look Like.
From jackwestin.com
Anion Cation Common Names Formulas And Charges For Familiar Ions Ions What Does A Cation Look Like Because one or more electrons are removed to. a cation is an ionic species with a positive charge. For example, in nacl, the sodium atom acts as the cation, while the. Explain how neutral atoms ionize to form cations. cations are ions that are positively charged. a cation is a positively charged ion with fewer electrons than. What Does A Cation Look Like.
From exogctlqx.blob.core.windows.net
Examples Of Not Soluble In Water at Edward Brafford blog What Does A Cation Look Like a cation is an ionic species with a positive charge. Ions are charged atoms or molecules. Cations are named according to the parent. Anions are ions that are negatively charged. when writing the formula of a compound, the cation is listed before the anion. The word cation comes from the greek. Groups 1 and 2 elements form cations.. What Does A Cation Look Like.
From circuitlibsmoring.z21.web.core.windows.net
Magnesium Ion Atom Diagram What Does A Cation Look Like Cations are named according to the parent. If a balanced atom loses one or more electrons, it will become a positively charged cation. Explain how neutral atoms ionize to form cations. The word cation comes from the greek. Anions are ions that are negatively charged. a cation is a positively charged ion with fewer electrons than protons [2] (e.g.. What Does A Cation Look Like.
From www.difference101.com
Cation vs. Anion 7 Key Differences, Pros & Cons, Examples Difference 101 What Does A Cation Look Like A cation is an ion that has lost one or more electrons, giving a net positive charge. Cations are named according to the parent. a cation is a positively charged ion with fewer electrons than protons [2] (e.g. If a balanced atom loses one or more electrons, it will become a positively charged cation. cations are formed by. What Does A Cation Look Like.
From www.youtube.com
Identifying Cations Reactions YouTube What Does A Cation Look Like cations are ions that are positively charged. Ions are charged atoms or molecules. If a balanced atom gains one or more electrons, it will become a negatively charged anion. when writing the formula of a compound, the cation is listed before the anion. A cation is an ion that has lost one or more electrons, giving a net. What Does A Cation Look Like.
From au.pinterest.com
Ion Names, Formulas and Charges Chart for Chemistry Classroom What Does A Cation Look Like Ions are charged atoms or molecules. K + (potassium ion)) while an anion is a negatively charged ion. Determine the charges achieved when main group. cations are formed by the loss of one or two electrons from an element. For example, in nacl, the sodium atom acts as the cation, while the. Anions are ions that are negatively charged.. What Does A Cation Look Like.
From chem.libretexts.org
2615 Qualitative Analysis of Selected Cations Chemistry LibreTexts What Does A Cation Look Like K + (potassium ion)) while an anion is a negatively charged ion. Anions are ions that are negatively charged. when writing the formula of a compound, the cation is listed before the anion. Determine the charges achieved when main group. The word cation comes from the greek. Groups 1 and 2 elements form cations. Cations are named according to. What Does A Cation Look Like.
From www.youtube.com
How to identify cations and anions in ionic compounds. YouTube What Does A Cation Look Like K + (potassium ion)) while an anion is a negatively charged ion. Explain how neutral atoms ionize to form cations. If a balanced atom loses one or more electrons, it will become a positively charged cation. Anions are ions that are negatively charged. Groups 1 and 2 elements form cations. Determine the charges achieved when main group. Ions are charged. What Does A Cation Look Like.
From www.youtube.com
Cations and Anions Explained YouTube What Does A Cation Look Like cations are ions that are positively charged. The word cation comes from the greek. a cation is a positively charged ion with fewer electrons than protons [2] (e.g. a cation is an ionic species with a positive charge. If a balanced atom gains one or more electrons, it will become a negatively charged anion. If a balanced. What Does A Cation Look Like.