Fda Drug Registration List . And a current inventory of all. Establishment registration and medical device listing files for download. Drug and biologic approval and ind activity reports. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Search the registration & listing database. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. Cder’s new molecular entities and new therapeutic biological products. This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s.
from www.cmaxinsight.com
Drug and biologic approval and ind activity reports. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Establishment registration and medical device listing files for download. This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. And a current inventory of all. Cder’s new molecular entities and new therapeutic biological products.
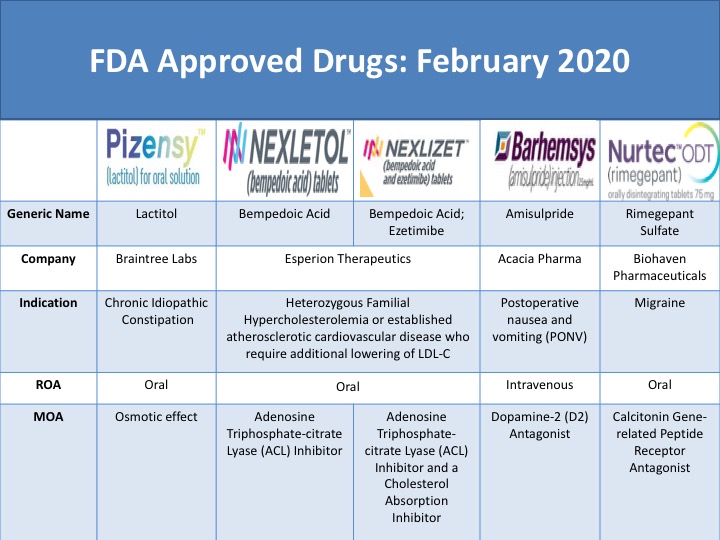
FDA Approved Drugs February 2020 CmaxInsight
Fda Drug Registration List And a current inventory of all. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: And a current inventory of all. This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s. Search the registration & listing database. Establishment registration and medical device listing files for download. Cder’s new molecular entities and new therapeutic biological products. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Drug and biologic approval and ind activity reports. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective.
From www.registrarcorp.com
How to Get FDA Approval Registrar Fda Drug Registration List Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Cder’s new molecular entities and new therapeutic. Fda Drug Registration List.
From www.meddevicecorp.com
US FDA Registration Service of Food, Drugs, Medical Devices Fda Drug Registration List Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. Establishment registration and medical device listing files for download. Search the registration & listing database. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. This information helps the fda maintain a catalog of all. Fda Drug Registration List.
From www.raslss.com
A Quick Overview on FDA Drug Approvals RAS LSS Fda Drug Registration List Drug and biologic approval and ind activity reports. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. Cder’s new molecular entities and new therapeutic biological products. This provides the agency with a list of. Fda Drug Registration List.
From www.fdahelp.us
How to Register with FDA FDAHELP.US Fda Drug Registration List There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. Search the registration & listing database. And a current inventory of all. Drug and biologic approval and ind activity reports. Cder’s new molecular entities. Fda Drug Registration List.
From www.fdahelp.us
How to Register with FDA FDAHELP.US Fda Drug Registration List There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Cder’s new molecular entities and new therapeutic biological products. Drug and biologic approval and ind activity reports. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. And a. Fda Drug Registration List.
From www.fdabasics.com
US FDA Registration is required for your product ? Understanding US FDA Fda Drug Registration List Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Cder’s new molecular entities and new therapeutic biological products. Fda uses science and data to ensure that approved drugs are of a high quality,. Fda Drug Registration List.
From www.slideshare.net
FDA Drug Registration Renewal and FDA Certificates Number Service F… Fda Drug Registration List Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. And a current inventory of all. This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s. Cder’s new molecular entities and new therapeutic biological products. There are three steps, or submissions, that are. Fda Drug Registration List.
From www.slideserve.com
PPT US Agent for FDA, OTC Registration FDA Company Registration Fda Drug Registration List And a current inventory of all. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Cder’s new molecular entities and new therapeutic biological products. Establishment registration and medical device listing files. Fda Drug Registration List.
From proper-cooking.info
Fda Certificate Fda Drug Registration List Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Cder’s new molecular entities and new therapeutic biological products. And a current inventory of all. The following charts detail the requirements. Fda Drug Registration List.
From www.fdalisting.com
Sample FDA Registration Certificates Fda Drug Registration List Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. And a current inventory of all. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. This. Fda Drug Registration List.
From www.28ceramics.com
Best Fda Registration Certificate Manufacture Fda Drug Registration List Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Establishment registration and medical device listing files for download. This information helps the fda maintain a catalog of all drugs and biologics in commercial. Fda Drug Registration List.
From www.slideserve.com
PPT FDA Registration and FDA Approval Process Online FDAhelp Fda Drug Registration List Search the registration & listing database. And a current inventory of all. Establishment registration and medical device listing files for download. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Drug and biologic approval and ind activity reports. This information helps the fda maintain a catalog of all. Fda Drug Registration List.
From www.researchgate.net
historical of Ms drug approval from Fda and eMa. Download Table Fda Drug Registration List Cder’s new molecular entities and new therapeutic biological products. Search the registration & listing database. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Drug and biologic approval and ind activity reports. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. This information helps the. Fda Drug Registration List.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Registration List Drug and biologic approval and ind activity reports. Establishment registration and medical device listing files for download. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Search the registration & listing database. Cder’s new molecular entities and new therapeutic biological products. Learn more about the fda’s role in reviewing,. Fda Drug Registration List.
From www.slideshare.net
Full us fda approved drug list Fda Drug Registration List Establishment registration and medical device listing files for download. And a current inventory of all. Drug and biologic approval and ind activity reports. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. Search the registration & listing database. There are three steps, or submissions, that are needed in order. Fda Drug Registration List.
From www.slideshare.net
FDA Drug Registration Renewal and FDA Certificates Number Service F… Fda Drug Registration List Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Cder’s new molecular entities and new therapeutic biological products. And a current inventory of all. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. The following charts detail the requirements for. Fda Drug Registration List.
From www.cmaxinsight.com
FDA Approved Drugs February 2020 CmaxInsight Fda Drug Registration List This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: The following charts detail. Fda Drug Registration List.
From www.akinglobal.com.tr
Akın Global Medical Fda Drug Registration List And a current inventory of all. Search the registration & listing database. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Cder’s new molecular entities and new therapeutic biological products. This. Fda Drug Registration List.
From medium.com
User Guide for FDA Food Facility Registration and FDA Registration Fda Drug Registration List Establishment registration and medical device listing files for download. Drug and biologic approval and ind activity reports. This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: This information helps. Fda Drug Registration List.
From ecommed.vn
The US Food and Drug Administration FDA Certificate Of Registration Fda Drug Registration List Establishment registration and medical device listing files for download. Search the registration & listing database. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. Drug and biologic approval and ind activity reports. This information. Fda Drug Registration List.
From digitalhealthcentral.com
Understand the differences between FDA Approved vs Cleared and Fda Drug Registration List Search the registration & listing database. Drug and biologic approval and ind activity reports. Establishment registration and medical device listing files for download. And a current inventory of all. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Fda uses science and data to ensure that approved drugs. Fda Drug Registration List.
From fontanaalmyconver.blogspot.com
Do I Need To Register With Fda To Get My Label Approved Fontana Fda Drug Registration List Cder’s new molecular entities and new therapeutic biological products. This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s. Search the registration & listing database. Drug and biologic approval and ind activity reports. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective.. Fda Drug Registration List.
From ecommed.vn
The US Food and Drug Administration FDA Certificate Of Registration Fda Drug Registration List Search the registration & listing database. Cder’s new molecular entities and new therapeutic biological products. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s. Drug and biologic approval and ind. Fda Drug Registration List.
From www.fdahelp.us
FDA Registration Number and other FDA Requirements FDAHELP.US Fda Drug Registration List Cder’s new molecular entities and new therapeutic biological products. This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s. Drug and biologic approval and ind activity reports. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: Establishment registration and. Fda Drug Registration List.
From crimsonlogic-northamerica.com
US Food and Drug Administration (FDA) Registration What You Need to Fda Drug Registration List Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Search the registration & listing database. Cder’s new molecular entities and new therapeutic biological products. Establishment registration and medical device listing files for download. There. Fda Drug Registration List.
From www.researchgate.net
List of Food and Drug Administration (FDA) approved AMPs for various Fda Drug Registration List Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Cder’s new molecular entities and new therapeutic biological products. Drug and biologic approval and ind activity reports. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. This information helps the fda. Fda Drug Registration List.
From www.libertymanagement.us
FDA Registration Number FDA Registration Search Fda Drug Registration List Cder’s new molecular entities and new therapeutic biological products. Establishment registration and medical device listing files for download. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: And a current inventory of all. The following charts detail the requirements for registration and listing based on the type of. Fda Drug Registration List.
From www.npssonipat.com
fda importer Fda Drug Registration List And a current inventory of all. Search the registration & listing database. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. Establishment registration and medical device listing files for download. Cder’s new molecular entities and new therapeutic biological products. Fda uses science and data to ensure that approved drugs are of a high quality,. Fda Drug Registration List.
From www.researchgate.net
List of FDAapproved drugs (2020) along with their results for Fda Drug Registration List Establishment registration and medical device listing files for download. Search the registration & listing database. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: And a current inventory of all. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. Cder’s new molecular. Fda Drug Registration List.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Drug Registration List And a current inventory of all. Establishment registration and medical device listing files for download. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. Drug and biologic approval and ind activity reports. Search the registration & listing database. Learn more about the fda’s role in reviewing, approving, and monitoring. Fda Drug Registration List.
From www.iascertification.com
US FDA Drug Registration Drug Registration Certificate Fda Drug Registration List Search the registration & listing database. This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Establishment registration and medical device listing files for download. Drug and biologic approval and ind activity reports. And a current inventory of all. Fda uses science and data to ensure that approved drugs are. Fda Drug Registration List.
From www.fda.gov
Electronic Drug Registration and Listing Using CDER Direct 10/22/2019 Fda Drug Registration List This information helps the fda maintain a catalog of all drugs and biologics in commercial distribution in the united states. Cder’s new molecular entities and new therapeutic biological products. The following charts detail the requirements for registration and listing based on the type of activity performed at that establishment. And a current inventory of all. Fda uses science and data. Fda Drug Registration List.
From www.i3cglobal.com
US FDA Registration Online in India 🥇(+91 99 45912081) Fda Drug Registration List This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s. There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: And a current inventory of all. Fda uses science and data to ensure that approved drugs are of a high. Fda Drug Registration List.
From biotechresearchgroup.com
General Facts about FDA Establishment Registration Tutorial Fda Drug Registration List Search the registration & listing database. Establishment registration and medical device listing files for download. Learn more about the fda’s role in reviewing, approving, and monitoring drugs in the latest. Cder’s new molecular entities and new therapeutic biological products. And a current inventory of all. Fda uses science and data to ensure that approved drugs are of a high quality,. Fda Drug Registration List.
From pharmaceuticalintelligence.com
2022 FDA Drug Approval List, 2022 Biological Approvals and Approved Fda Drug Registration List There are three steps, or submissions, that are needed in order to register an establishment and list a drug with fda: And a current inventory of all. This provides the agency with a list of all drug manufacturers currently producing drugs for sale in the u.s. The following charts detail the requirements for registration and listing based on the type. Fda Drug Registration List.