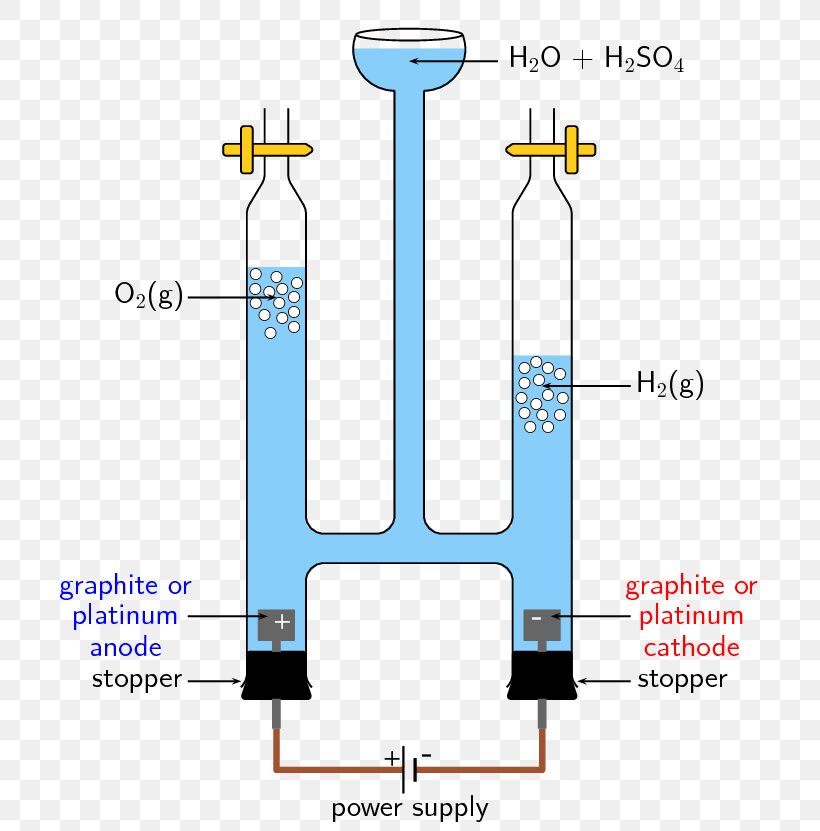
Water Electrolysis Equations . Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. The electrolysis of water produces hydrogen and oxygen gases. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The ions move to the opposite electrodes to liberate. In this section, we look at how. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: An external voltage is applied to drive a nonspontaneous reaction. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:.
from narodnatribuna.info
During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: In this section, we look at how. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. The electrolysis of water produces hydrogen and oxygen gases. The ions move to the opposite electrodes to liberate. An external voltage is applied to drive a nonspontaneous reaction.
Draw A Neat Diagram Of Electrolysis Of Water And Explain
Water Electrolysis Equations In an electrolytic cell, however, the opposite process, called electrolysis, occurs: In this section, we look at how. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolysis of water produces hydrogen and oxygen gases. An external voltage is applied to drive a nonspontaneous reaction. The ions move to the opposite electrodes to liberate. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added.
From www.nagwa.com
Question Video Identifying the Correct Equation for the Electrolysis Water Electrolysis Equations In this section, we look at how. The electrolysis of water produces hydrogen and oxygen gases. An external voltage is applied to drive a nonspontaneous reaction. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: This chemistry video tutorial provides a basic introduction into the electrolysis of water which. The electrolytic cell consists of. Water Electrolysis Equations.
From www.youtube.com
Electrolysis of Water YouTube Water Electrolysis Equations The ions move to the opposite electrodes to liberate. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. The electrolysis of water produces hydrogen and oxygen gases. This chemistry video tutorial provides a basic introduction into the electrolysis. Water Electrolysis Equations.
From enagic-asia.com
The electrolysis process Enagic Kangen Water Water Electrolysis Equations Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: An external voltage is applied to drive a nonspontaneous reaction. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: This chemistry video tutorial provides a basic introduction. Water Electrolysis Equations.
From enginewiringsmall.z19.web.core.windows.net
Circuit Diagram For Electrolysis Of Water Water Electrolysis Equations The ions move to the opposite electrodes to liberate. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. In this section, we look at how. This chemistry video tutorial provides a basic introduction into the electrolysis. Water Electrolysis Equations.
From www.hotelsrate.org
Water Electrolysis Equation Modern Home Designs Water Electrolysis Equations An external voltage is applied to drive a nonspontaneous reaction. The electrolysis of water produces hydrogen and oxygen gases. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. In this section, we look at how. The. Water Electrolysis Equations.
From www.youtube.com
Electrolysis of Water Electrochemistry YouTube Water Electrolysis Equations During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The ions move to the opposite electrodes to liberate. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: This chemistry video tutorial provides a basic introduction. Water Electrolysis Equations.
From www.slideserve.com
PPT Lecture 41 Electrochemistry V PowerPoint Presentation, free Water Electrolysis Equations The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. In this section, we look at how. The ions move to the. Water Electrolysis Equations.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID2216212 Water Electrolysis Equations The electrolysis of water produces hydrogen and oxygen gases. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. An external voltage is applied to drive a nonspontaneous reaction. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. In an electrolytic cell, however, the opposite process, called electrolysis,. Water Electrolysis Equations.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Water Electrolysis Equations This chemistry video tutorial provides a basic introduction into the electrolysis of water which. In this section, we look at how. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. In an electrolytic cell, however, the opposite process,. Water Electrolysis Equations.
From study.com
Electrolysis of Aqueous Solutions Lesson Water Electrolysis Equations The ions move to the opposite electrodes to liberate. An external voltage is applied to drive a nonspontaneous reaction. The electrolysis of water produces hydrogen and oxygen gases. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water. Water Electrolysis Equations.
From www.youtube.com
Electrolysis of water Chemical reactions and Equations LabTurtle Water Electrolysis Equations This chemistry video tutorial provides a basic introduction into the electrolysis of water which. An external voltage is applied to drive a nonspontaneous reaction. The electrolysis of water produces hydrogen and oxygen gases. The ions move to the opposite electrodes to liberate. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water. Water Electrolysis Equations.
From www.tessshebaylo.com
Water Electrolysis Balanced Equation Tessshebaylo Water Electrolysis Equations An external voltage is applied to drive a nonspontaneous reaction. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. In this section, we look at how. Water electrolysis is divided into three categories depending on the charge carrier and. Water Electrolysis Equations.
From www.hotelsrate.org
Water Electrolysis Equation Diy Projects Water Electrolysis Equations Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. The ions move to the opposite electrodes to liberate. An external voltage is applied to drive a nonspontaneous reaction. In an electrolytic cell, however, the opposite process, called. Water Electrolysis Equations.
From water.lsbu.ac.uk
Electrolysis of water Water Electrolysis Equations During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: This chemistry video tutorial provides a basic introduction into the electrolysis of water which. The electrolysis. Water Electrolysis Equations.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Water Electrolysis Equations In this section, we look at how. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: An external voltage is applied to drive a nonspontaneous reaction. The ions move to the opposite electrodes to liberate. The electrolysis of water produces hydrogen and oxygen. Water Electrolysis Equations.
From narodnatribuna.info
Draw A Neat Diagram Of Electrolysis Of Water And Explain Water Electrolysis Equations During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. An external voltage is applied to drive a nonspontaneous reaction. The electrolysis of. Water Electrolysis Equations.
From scienceinfo.com
Electrolysis of Water Definition, Principle, and Applications Water Electrolysis Equations During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. The electrolysis of water produces hydrogen and oxygen gases. The ions move to the opposite electrodes to liberate. In this section, we look at how. An external voltage is. Water Electrolysis Equations.
From www.youtube.com
WATER ELECTROLYSIS DEMONSTRATION WITH EXPLANATION CHEMISTRY GRADE 8 Water Electrolysis Equations In this section, we look at how. The electrolysis of water produces hydrogen and oxygen gases. The ions move to the opposite electrodes to liberate. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolytic cell consists of a pair of platinum electrodes. Water Electrolysis Equations.
From www.teachoo.com
Chemical Equation Meaning, How to Write [with 5+ Examples] Teachoo Water Electrolysis Equations Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so. Water Electrolysis Equations.
From www.shutterstock.com
2 Electrolysis Of Water Equation Images, Stock Photos & Vectors Water Electrolysis Equations The electrolysis of water produces hydrogen and oxygen gases. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. The ions move to. Water Electrolysis Equations.
From www.tessshebaylo.com
Water Electrolysis Half Equations Tessshebaylo Water Electrolysis Equations Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: In this section, we look at how. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such. Water Electrolysis Equations.
From www.tessshebaylo.com
Water Electrolysis Equation Tessshebaylo Water Electrolysis Equations An external voltage is applied to drive a nonspontaneous reaction. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4. Water Electrolysis Equations.
From www.youtube.com
Electrolysis of water (Chemical equation and reactions) Water Electrolysis Equations During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. In this section, we look at how. The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so. Water Electrolysis Equations.
From byjus.com
Explain the electrolysis of water with the help of diagram. Water Electrolysis Equations Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. The electrolysis of water produces hydrogen and oxygen gases. The ions move to the opposite electrodes to liberate. In this section, we look at how. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. An external voltage is applied. Water Electrolysis Equations.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Circuit Diagram For Electrolysis Of Water Water Electrolysis Equations In this section, we look at how. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The ions move to the opposite electrodes to liberate. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has. Water Electrolysis Equations.
From www.researchgate.net
Schematic illustration of (a) traditional water electrolysis and (b Water Electrolysis Equations During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4. Water Electrolysis Equations.
From www.chemistrylearner.com
Electrolysis of Water Definition and Equation Water Electrolysis Equations The ions move to the opposite electrodes to liberate. Water may be electrolytically decomposed in a cell similar to the one illustrated in figure 17.19. In this section, we look at how. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4. Water Electrolysis Equations.
From www.tes.com
Electrolysis of Aqueous Solutions and Half Reactions Edexcel 91 Water Electrolysis Equations In this section, we look at how. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolysis of water produces hydrogen and oxygen gases. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. The ions move to the opposite electrodes to liberate. The electrolytic cell consists of a. Water Electrolysis Equations.
From www.youtube.com
The Electrolysis Of Water (GCSE Chemistry) YouTube Water Electrolysis Equations The ions move to the opposite electrodes to liberate. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: An external voltage is applied to drive a nonspontaneous reaction. This chemistry video tutorial provides a basic introduction into. Water Electrolysis Equations.
From www.slideshare.net
6.3 (a) electrolysis of an aqueous solution Water Electrolysis Equations The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. Water may be electrolytically decomposed in a cell similar to the one. Water Electrolysis Equations.
From www.teachoo.com
MCQ Class 10 Electrolysis of water is a reaction. The Water Electrolysis Equations An external voltage is applied to drive a nonspontaneous reaction. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: During the electrolysis of. Water Electrolysis Equations.
From www.pinterest.com
Electrolysis of Water Definition and Equation Water electrolysis Water Electrolysis Equations In this section, we look at how. This chemistry video tutorial provides a basic introduction into the electrolysis of water which. The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Water may be electrolytically decomposed in a cell similar to the one illustrated in figure. Water Electrolysis Equations.
From manualdefecation.z21.web.core.windows.net
Electrolysis Of Water Diagram Water Electrolysis Equations An external voltage is applied to drive a nonspontaneous reaction. The ions move to the opposite electrodes to liberate. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: This chemistry video tutorial provides a basic introduction into the electrolysis of water which. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a. Water Electrolysis Equations.
From www.sscadda.com
Electrolysis of Water Equation, Diagram and Experiment Water Electrolysis Equations The ions move to the opposite electrodes to liberate. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. In this section, we look at how. Water electrolysis is divided into three categories depending on the charge carrier and. Water Electrolysis Equations.
From morrisclassicalacademy.blogspot.com
Morris Classical Academy Electrolysis of water Water Electrolysis Equations The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: During the electrolysis of water, hydrogen and oxygen gases are produced according to the following chemical equation:. This. Water Electrolysis Equations.